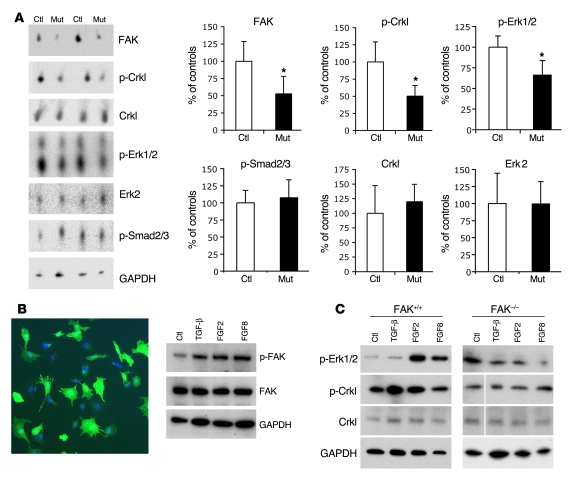
Figure 6. FAK-dependent Crkl and Erk1/2 phosphorylation in NCCs.
(A) Decreased Crkl and Erk1/2 phosphorylation in mutant outflow tracts. Western blot analysis of control and mutant outflow tract samples showing significant decrease of FAK levels (52.74% ± 25.37%; n = 4), phospho-Crkl levels (p-Crkl levels, 50.24% ± 18.78%), and phopho-Erk1/2 (p-Erk1/2 levels, 66.19% ± 17.56%) in mutants compared with control littermates (100%; n = 4; *P ≤ 0.05). Total levels of Crkl, Erk2, phospho-Smad2/3 (p-Smad2/3), and GAPDH are not significantly changed. Statistical significance was determined using nonpaired, 2-tailed Student’s t test. Data are expressed as mean ± SD. (B) Increased FAK phosphorylation in NCCs in vitro after addition of growth factors. Photograph illustrating an in vitro NCC culture stained with GFP-488 to show recombinant NCC cells from Wnt1CreZ/EG embryos and DAPI for nuclear staining (left panel). Original magnification, ×100. Western blot analysis reveals increased FAK phosphorylation in NCCs in vitro after 30 minutes of incubation with different growth factors (TGF-β, FGF2, and FGF8) compared with nontreated control cells (right panel). Three independent experiments yielded similar results. (C) Increased Erk1/2 and Crkl phosphorylation are observed in wild-type NCCs after addition of FGF2/FGF8 and TGF-β/FGF2, respectively. No obvious changes in Erk1/2 or Crkl phosphorylation are observed in Fak-deficient NCCs after growth factor addition. White lines indicate noncontiguous lanes run on the same gel.