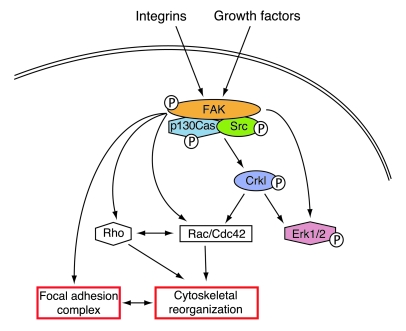
Figure 7. Model for FAK requirement in NCCs during cardiovascular development.
FAK signaling is required by postmigratory NCCs for appropriate cell function. In NCCs, FAK is phosphorylated by integrin activation and growth factors, such as TGF-β and FGFs. Fak-deficient NCCs are unable to propagate or integrate certain integrin and growth factor signals. Specifically, during outflow tract septation, Crkl and Erk1/2 phosphorylation is impaired in Fak-deficient NCCs, leading to cardiac developmental defects that recapitulate DiGeorge syndrome. Deficient Crkl phosphorylation potentially affects Crkl binding ability, through its SH2 domain, to other proteins, such as paxillin and p130CAS, and translocation of Crkl to focal adhesions. Therefore, activation of Rac1 and Cdc42 is potentially affected in Fak-deficient NCCs, which results in reduced cortactin localization to the cell periphery, abnormal cell morphology, and cytoskeletal reorganization.