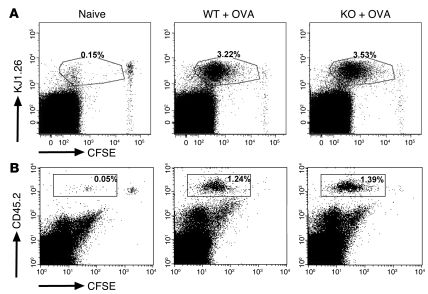
Figure 6. Loss of EBAG9 does not affect the proliferation of naive, OVA-specific transgenic T cells in vivo.
(A) CFSE-labeled naive CD4+ DO11.10 T cells (1 × 106) were adoptively transferred into Ebag9–/– and Ebag9+/+ recipients (Balb/c, H-2d haplotype). Eighteen hours later, mice were immunized by s.c. injection of 100 μg OVA protein and 25 μg CpGs. Control WT and KO animals received T cells but no OVA protein (naive). Proliferation of transferred T cells in draining lymph nodes was analyzed by flow cytometry at day 3. Representative FACS plots of gated CD4+ cells from pooled draining lymph nodes (WT, n = 4; KO, n = 5 mice). Numbers indicate the percentage of proliferated CFSE-labeled KJ1.26+ responder T cells in WT or KO recipients within the gate. No differential stimulation of responder T cells was observed in WT or KO recipients. (B) Naive OT-I/Ebag9–/– (n = 4 mice) and OT-I/Ebag9+/+ CD8+ (n = 4 mice) T cells (CD45.2 congenic mouse strain) were CFSE labeled and transferred i.v. into congenic (CD45.1 congenic mouse strain) recipients (5 × 105 cells). Twenty hours later, animals were immunized i.v. with OVA protein (1 μg/g body weight) and CpGs (25 μg). Control groups included a non-immunized animal. Two days later, proliferation of transferred T cells in spleen was analyzed by flow cytometry. Transferred T cells were detected by anti-CD45.2 (LY-5.2) staining. Numbers indicate the percentage of proliferated CFSE-labeled CD45.2+ responder T cells in WT or KO recipients within the gate. Shown are representative data for 5 congenic recipients of OT-I/Ebag9–/– T cells and 4 congenic recipients of OT-I/Ebag9+/+ T cells.