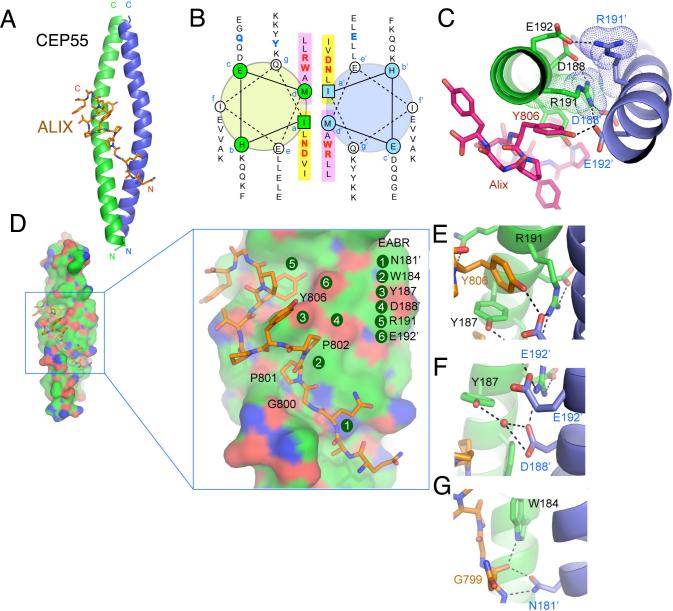
Figure 2. Structure of the non-canonical CEP55-EABR coiled coil and its complex with ALIX.
(A) The overall structure of the CEP55-EABR homodimer (green and blue ribbons) in complex with the ALIX peptide (stick model, carbon orange, oxygen red, nitrogen blue). At right, the intercoil Cα-Cα distance at the a and d positions of the coiled-coil is shown as a function of residue number for CEP55 (red curve) as compared to the average Cα-Cα distance at the a and d positions of the GCN4 leucine zipper (blueline). (B) Helical wheel analysis of the six heptad repeats of the EABR coiled coil. (C) Charge repulsion between Asp188, Arg191, and Glu192 pairs in the homodimer creates asymmetry in the coiled coil and interactions with the ALIX peptide. (D) Overview and close-ups of selected regions of the CEP55 surface (carbon green, oxygen red, nitrogen blue), with the ALIX peptide colored as in (A). (E-G) Molecular interactions in the complex shown in detail and colored as in (A).