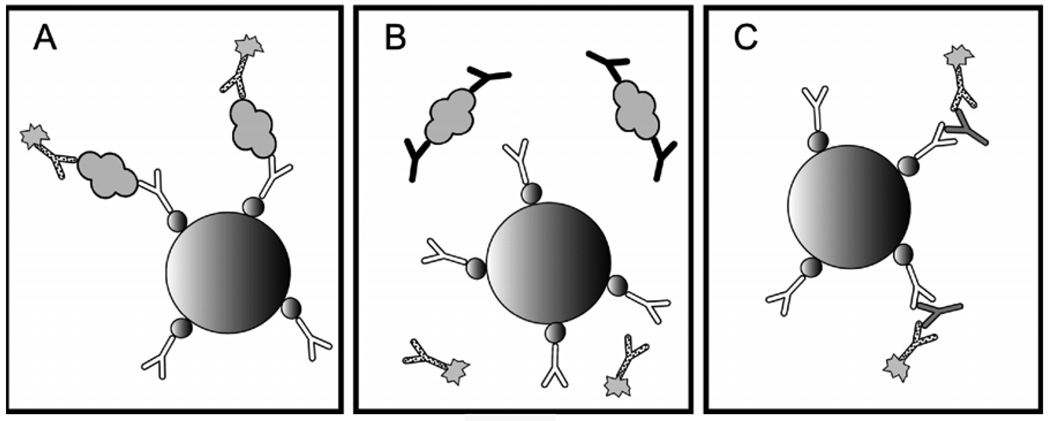
Figure 1. Endogenous antibody interference in sandwich immunoassays.
A modern sandwich immunoassay for thyroglobulin is pictured on the left. A streptavidin coated paramagnetic bead (large sphere) binds biotin-labeled (small spheres) capture antibody (white), which forms a sandwich with analyte (gray globule) and enzyme-labeled (sunburst) reporter antibody (dotted). Sandwiches bound to beads are separated using a magnet, unbound reporter antibody is washed away, and enzyme is detected using one of a variety of automated methodologies. Unfortunately, in a high percentage of patients, the epitopes needed for sandwich formation are sterically protected by anti-analyte antibodies (black) as pictured in the middle. In other patients, non-specific anti-reagent antibodies (gray) are able to bridge the gap between capture and reporter antibodies in the absence of analyte as picture on the right.