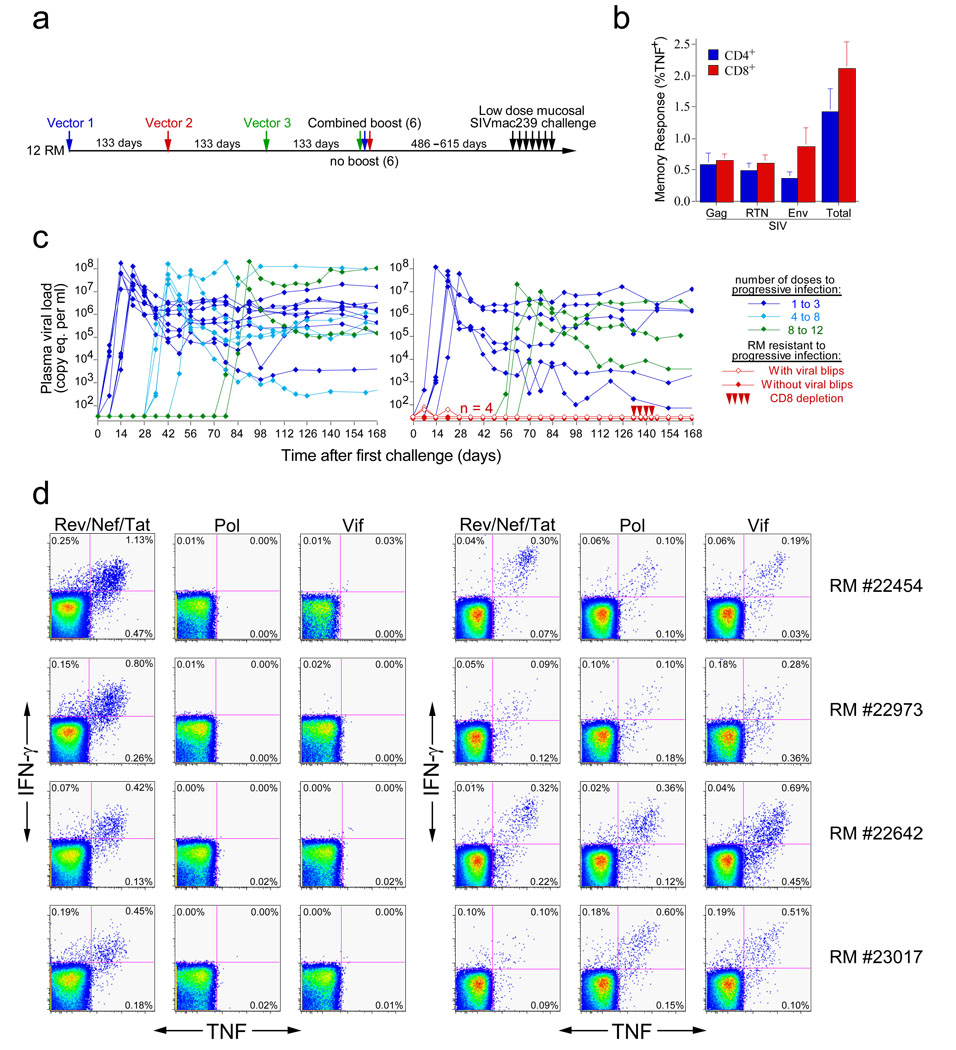
Figure 4. RM inoculated with RhCMV vectors expressing Gag, Rev/Nef/tat, and Env are protected from progressive SIVmac239 infection following repeated, limiting dose intra-rectal challenge.
(a) Vaccination and challenge protocol for efficacy assessment of RhCMV vectors. RhCMV/Gag, RhCMV/Retanef, and RhCMV/Env vectors were given individually at 133 day intervals in the following orders: 1) Gag/Retanef/Env, 2) Retanef/Env/Gag, and 3) Env/Gag/Retanef for 4 RM each. Half of each group was subsequently provided with a combined boost of all 3 vectors. The long-term anti-SIV T cell responses did not differ between these sub-groups, and all 12 of these RM were therefore combined into one vaccinated group for challenge (in comparison to 16 unvaccinated, but RhCMV+, control RM). (b) Mean pre-challenge frequencies (± SEM) of RhCMV-vectored, SIV Gag-, Rev/Nef/Tat-, and Env-specific responses among blood CD4+ and CD8+ memory T cells of the 12 vaccinated RM. (c) Plasma viral loads of the control (left panel) and vaccinated (right panel) RM cohorts over the course of, and subsequent to, limiting dose intra-rectal SIVmac239 challenge. Four of 12 vaccinated RM resisted progressive infection: these protected RM were treated with the humanized anti-CD8 mAb cMT807 at days 133, 136, 140 and 143 post-initial challenge (10, 5, 5, and 5 mg/kg doses, respectively) and were profoundly depleted of CD8+ T cells from blood (< 2.5% of baseline absolute counts) for 14 to 21 days. (d) FCICA of peripheral blood CD8+ T cells from the 4 protected vaccinees examining the response of these cells to SIV proteins that were (Rev/Nef/Tat) or were not (Pol and Vif) expressed by the administered RhCMV vectors, before (right panel) and 133 days after (left panel) initiation of SIVmac239 intra-rectal challenge protocol.