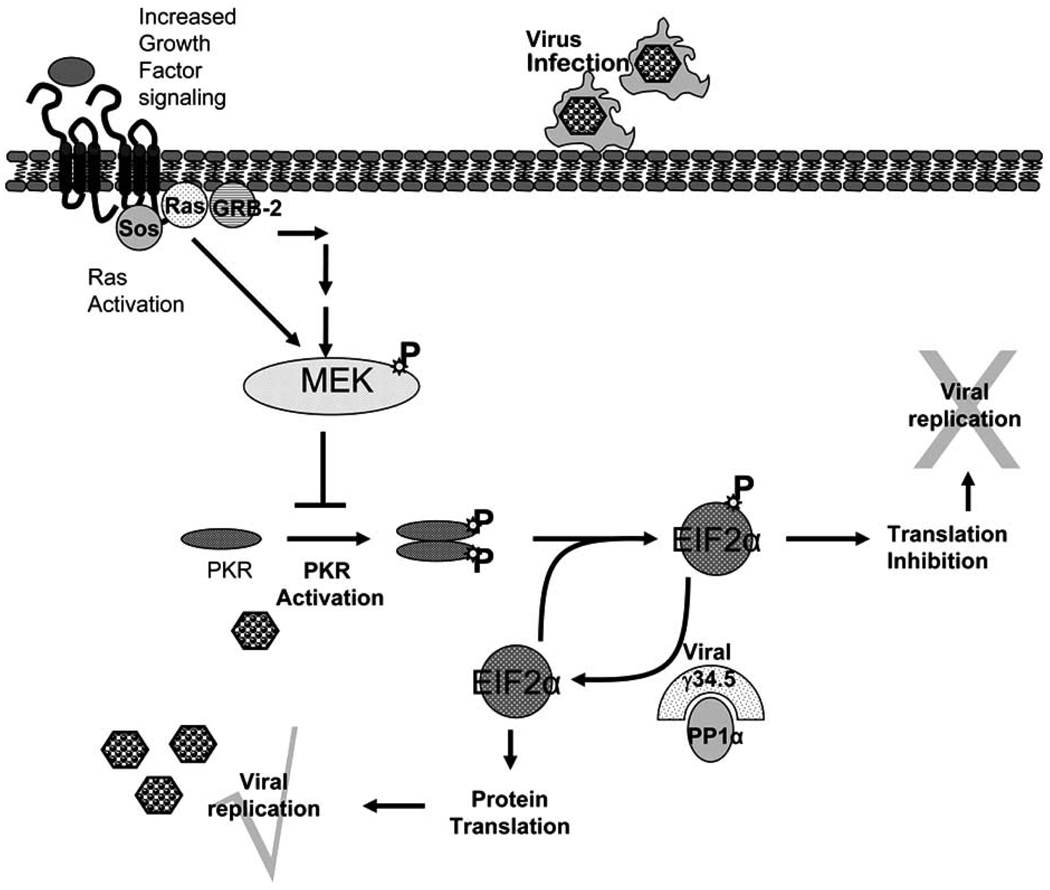
Fig. 2.
Viral infection of cells initiates the homo-dimerization and subsequent phosphorylation and activation of interferon-induced, double-stranded RNA-activated protein kinase (PKR). Activated PKR phosphorylates its natural substrate, the alpha subunit of eukaryotic protein synthesis initiation factor-2 (EIF2-alpha), leading to the inhibition of cellular protein synthesis. HSV-1 encodes for γ34.5, a viral protein that mediated protein phosphatase 1α to dephosphorylate EIF2-alpha, releasing the translational block initiated in normal cells upon infection. In the absence of viral γ34.5 gene the viral replication is blocked by cellular responses in normal cells. Cancer cells with an activated RAS/MEK pathway suppress PKR autophosphorylation, hence allowing γ34.5−/− virus to replicate in them.