Figure 2. p110α gatekeeper mutants have reduced catalytic activity.
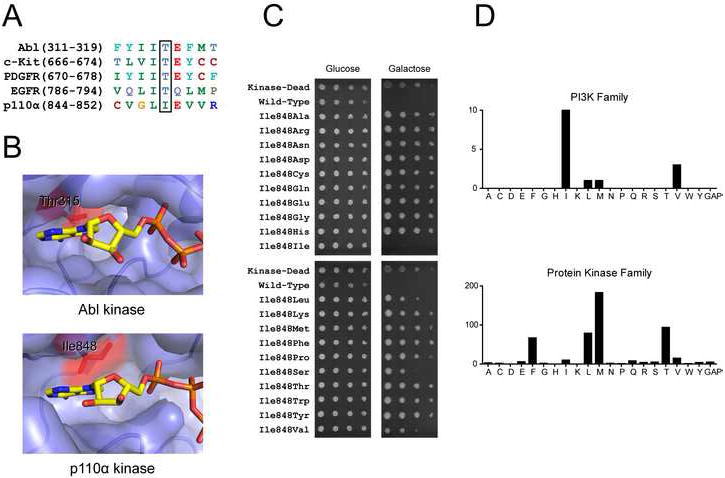
A: Sequence alignment of p110α with protein kinases that display clinical resistance mutations at the gatekeeper position
B: ATP binding sites of Abl (PDB code 2G1T) and p110α (PDB code 2RD0). ATP from p110γ co-crystal (PDB code 1E8X) was overlaid onto the apo p110α structure by structural alignment. The gatekeeper residue is colored red and labeled in both structures.
C: Six-fold serial dilutions of YRP1-pURA3-2μ-GAL1-p110αH1047R-CAAX strains with the indicated p110α mutations were spotted onto agar plates of SD −URA media containing either glucose or galactose. The plates were incubated at 30°C for 2 days (glucose) and 6 days (galactose).
D: Distribution of residues at the gatekeeper position in the human PI3K and protein kinase families.
