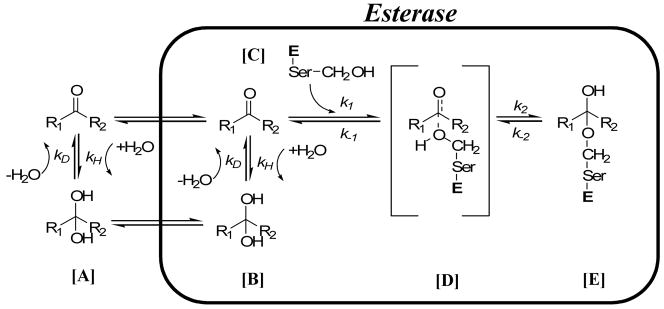
Figure 2.
Potential inhibition pathway for esterases. In [A], the TFK inhibitor establishes an equilibrium between the ketone and gem-diol forms. This equilibrium is affected by the R1 and R2 moieties and can be described by the rate constants for hydration (kH) and dehydration (kD). The equilibrium tends to be shifted towards the ketone for potent inhibitors (i.e, kD is greater). The ketone or gem-diol, or potentially both, then diffuse into the enzyme. It is hypothesized that the ketone is the active form of the inhibitor. Accordingly, if the gem-diol form of the inhibitor diffuses into the active site, it needs to undergo a dehydration step from [B] to form the ketone in [C]. This dehydration step can again be described by the rate constants for hydration (kH) and dehydration (kD). The ketone then undergoes nucleophilic attack by the catalytic serine residue forming the transient intermediate in [D] according to the rate constant k1. This intermediate can either collapse as described by k−1, releasing the ketone, or form the hemiketal enzyme/inhibitor complex in [E] according to the rate constant k2. The “Ser” indicates the catalytic serine residue and “E” is esterase.