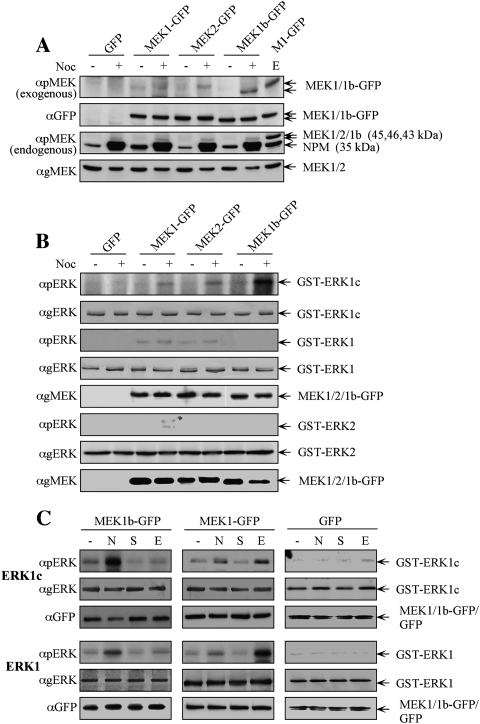
Figure 1.
ERK1c phosphorylation in mitosis is preferentially mediated by the alternative spliced MEK1b. (A) MEK1b undergoes phosphorylation during mitosis. HeLa cells were transfected with GFP, MEK1-GFP, MEK2-GFP, or MEK1b-GFP. Each of these transfected cells was treated as follows: untreated (−), nocodazole [100 ng/mL, 24 h (+)], or serum-starved; and then stimulated with EGF [50 ng/mL, 3 min (E)]. The cells were harvested and subjected to Western blotting with anti-pMEK, anti-GFP, and anti-gMEK(C-18) Abs. (B) MEK1b preferentially phosphorylates ERK1c during mitosis. HeLa cells were transfected as in A. The cells were treated with nocodazole [100 ng/mL, 24 h (+)] or left untreated (−). The cells were then harvested, and the GFP proteins were immunoprecipitated with anti-GFP Ab. The precipitated proteins were subjected to an in vitro phosphorylation system using GST-ERK1, 2 and 1c as substrates, as described in the Materials and Methods. The amounts of ERK1, 2 and 1c as well as their phosphorylation levels were determined by Western blotting using αpERK, and αgERK(CRS) Abs. The relative amounts of MEKs were determined by anti-gMEK(C-18) Ab, the MEKs used to phosphorylate ERK1/1c are in panel 5, and for ERK2 are in panel 8. (C) Differences between MEK1b and MEK1 stimulation and substrate determination. HeLa cells were transfected with MEK1b-GFP, MEK1-GFP, or GFP. The cells were treated as follows: untreated (−), nocodazole [100 ng/mL, 24 h (N)], serum-starved [16 h 0.1% FCS, (S)], or serum-starved and then stimulated with EGF [50 ng/mL, 3 min (E)]. The cells were harvested and the GFP-tagged proteins were immunoprecipitated by αGFP Ab, and each one of them was subjected to an in vitro phosphorylation using GST-ERK1/1c as substrates. The amounts of ERK1/1c as well as their phosphorylation levels were determined by Western blotting using anti-pERK, anti-gERK(CRS), and anti-GFP Abs.