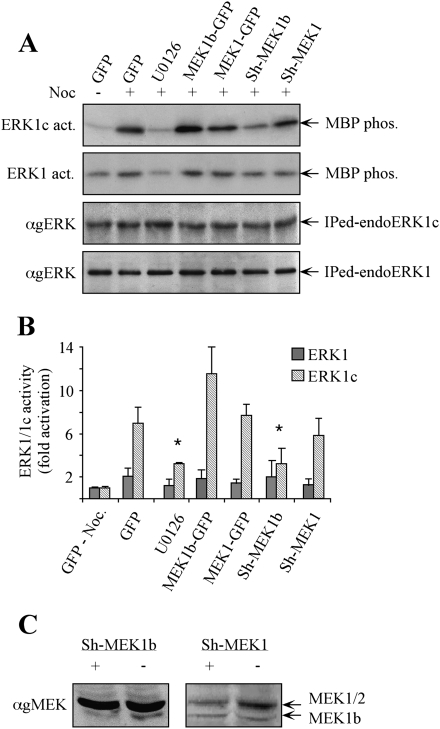
Figure 4.
Modulation of MEK1b expression affects nocodazole-dependent ERK1c activity in vivo. (A) HeLa cells were transfected as follows: GFP, MEK1b-GFP, MEK1-GFP, Sh-MEK1b, and Sh-MEK1. The cells were treated with nocodazole [100 ng/mL, 24 h, (+)]. The MEK inhibitor U0126 (5 μM, 1 h) was added prior to the cell harvesting. The cells were harvested, and endogenous ERK1 or ERK1c was immunoprecipitated. The precipitated proteins were subjected to in vitro phosphorylation using MBP as a substrate (ERK1c and ERK1 act.). The amounts of immunopreipitated endogenous ERKs (immunoprecipitated endoERK1 and ERK1c) were determined by Western blotting using anti-gERK(CRS) Ab. (B) Quantification of kinase activation. Fold activations of ERK1c and ERK1 were quantified by densitometry and presented as means ± SE of three experiments. All bar-graphs, except of the first two, represent stimulation with nocodazole. A Student's t-test was used to determine statistical significance ([*] P < 0.05). (C) Specific knockdown of MEK1b and MEK1 by their corresponding Sh-RNAs. HeLa cells were transfected with a plasmid containing Sh-RNA of MEK1b (Sh-MEK1b, +), MEK1 (Sh-MEK1, +), or a control plasmid (−). Forty-eight hours after transfection, the cells were harvested and subjected to SDS-PAGE and Western blotting with anti-gMEK (H-8) Abs.