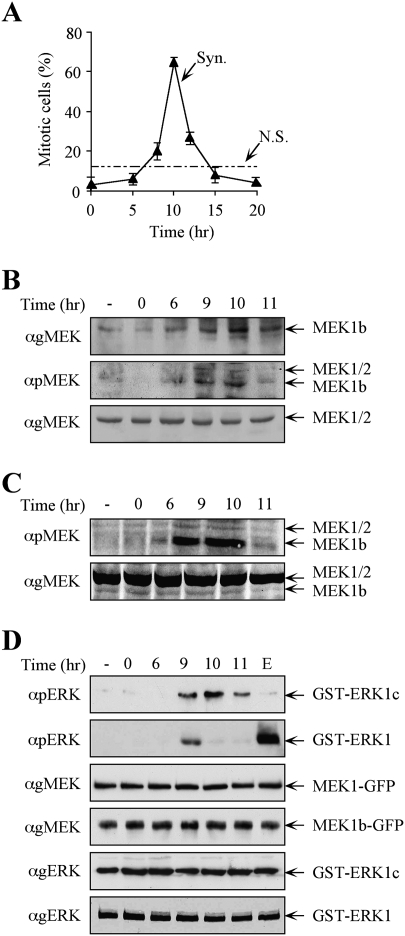
Figure 5.
The expression levels and activity of MEK1b are elevated during mitosis. HeLa cells were synchronized by a double-thymidine block, followed by the removal of the block to enable cell cycle progression (Syn), or left untreated (nonsynchronized, N.S.) as a control. (A) Mitotic index determination. The cells were fixed and stained with DAPI. The percentage of mitotic cells out of the total number of nuclei counted is presented as the means ± SE of three experiments; N = 300. (B) MEK1b expression and phosphorylation are elevated in the G2/M phase of the cell cycle. The cells were harvested and subjected to Western blotting with anti-gMEK(H-8) and anti-pMEK Abs. For a better resolution of the MEK1b, SDS-PAGE was longer than in previous experiments. Exposure times for gMEK1/2 and gMEK1b were different. (C) Specific MEK1b phsphorylation is elevated during mitosis. Equal amounts of MEK1b (judged by a calibrating Western blot) from each time after block release were subjected to Western blotting with anti-pMEK Ab. (D) MEK1b activity is enhanced in the G2/M phase of the cell cycle. HeLa cells were transfected with MEK1b-GFP or MEK1-GFP. The cells were synchronized by double-thymidine block, and times after release are indicated. In addition, one plate was serum-starved and stimulated with EGF (50 ng/mL, 3 min; E). The cells were harvested, and MEK1b or MEK1 was immunoprecipitated using anti-GFP Ab. The immunoprecipitated proteins were used as kinases for in vitro phosphorylation, where GST-ERK1c served as a substrate for MEK1b and GST-ERK1 for MEK1. The amounts of ERK1/1c as well as their phosphorylation levels were determined by Western blotting using anti-pERK, anti-gMEK(H-8), and anti-gERK(CRS) Abs.