Abstract
We describe the initial—and successful—use of the TandemHeart System's catheters to provide extracorporeal membrane oxygenation (ECMO), in 2 patients. In 1 patient, who was experiencing severe primary respiratory failure, the catheters provided a standard venovenous ECMO circuit. In the other patient, who had severe, acute pulmonary hypertension and right-heart failure, the catheters enabled a novel right atrial-to-left atrial circuit for ECMO. We discuss the potential of the TandemHeart System's catheters to provide novel and possibly superior vascular routes for the delivery of ECMO in different types of cardiopulmonary failure.
Key words: Extracorporeal circulation; extracorporeal membrane oxygenation/instrumentation/methods; heart failure/therapy; heart-assist devices; hemodynamics; methods; pneumonia/complications; respiratory insufficiency/therapy; technology assessment, biomedical; treatment outcome
Extracorporeal membrane oxygenation (ECMO) first came into wide clinical use as a tool to enable open-heart surgery through cardiopulmonary bypass (CPB) in the 1960s.1,2 By the 1970s, there was hope that ECMO technology could provide effective temporary oxygenation in patients who had a major, yet reversible, lung injury but in whom mechanical ventilation was not completely effective.3 A generation later, ECMO technology is a well-established, widely used method of support in neonatal and pediatric patients who experience severe respiratory failure.4 In contrast, progress in the application of ECMO to improve outcomes of respiratory failure in adults has been much slower. A large National Institutes of Health-sponsored trial of ECMO use in severe respiratory failure of various causes5 failed to show a benefit and thereby dampened enthusiasm for this application for more than a decade. New technology and implementation methods, however, have reinvigorated interest in the use of ECMO when severe respiratory failure occurs in adults.6,7 Indeed, data from the recently completed, large, prospective CESAR trial suggest that ECMO may already be of great usefulness in patients who are experiencing severe respiratory failure.8–10
There have also been encouraging reports of the successful use of ECMO to support patients who are experiencing acute right-heart failure,11 acute refractory left-heart failure,12 postcardiotomy heart failure,13 prolonged cardiac arrest,14 and postoperative cardiogenic shock.15 In these circumstances, ECMO has served temporarily (sometimes along with mechanical hemodynamic support) until heart recovery, or as a bridge to heart transplantation, heart–lung transplantation, or the placement of a left ventricular (LV) assist device.
The TandemHeart® PTVA® System (CardiacAssist, Inc.; Pittsburgh, Pa) is a new, percutaneously placed, ventricular assist device that has proved to be effective in the short-term management of acute heart failure.16 The system features innovative vascular-access catheters that enable a minimally invasive approach to mechanical LV assistance. We report here the 1st experience of using these catheters to perform ECMO, in 2 patients. One patient had severe primary respiratory failure, and the other had respiratory failure and right-heart failure caused by severe pulmonary hypertension. The TandemHeart catheters were placed in different intravascular locations in each patient. Our experience suggests that the TandemHeart catheter system can offer innovative and superior options for ECMO delivery to different populations of patients who experience cardiopulmonary failure.
Case Reports
Patient 1
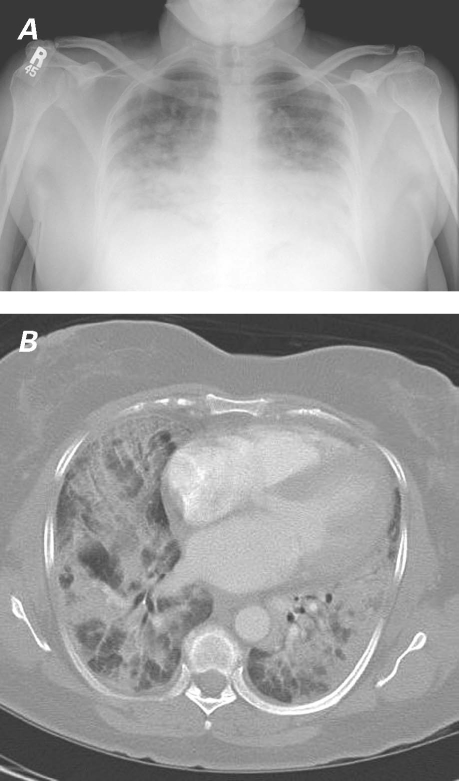
In February 2006, a 59-year-old woman presented with cough and progressive dyspnea of 2 weeks' duration. Her medical history was notable only for mild hypertension. Laboratory tests, including multiple serologic studies, revealed nothing unusual. The only abnormality upon physical examination was diffuse inspiratory crackles, most pronounced at the lung bases. Figure 1 shows the chest radiograph and computed tomogram upon presentation. The patient was initially diagnosed with pneumonia, but when empiric antibiotics led to no improvement, she underwent bronchoscopy with bronchoalveolar lavage and transbronchial biopsy. Because these procedures failed to yield a microbiologic or other clear diagnosis, she underwent a video-assisted thoracoscopic lung biopsy. The result of the biopsy showed classical signs of chronic organizing pneumonia (Fig. 2).
Fig. 1 Patient 1. A) Chest radiograph and B) illustrative section from chest computed tomogram show pneumonia.
Fig. 2 Patient 1. A) Low-power (H & E, orig. ×4) and B) high-power (H & E, orig. ×40) histograms of a lung biopsy show classical findings of chronic organizing pneumonia.
Chronic organizing pneumonia is a primary inflammatory disease of the lungs. In most patients, it fully responds to steroidal therapy.17 Accordingly, as soon as the biopsy report became available, our patient was started on high-dose intravenous steroidal agents (methylprednisone, 1 g/day) on the 1st postoperative day, while she was still undergoing mechanical ventilation. However, despite the steroids and maximal ventilator support, her oxygenation progressively worsened over the next 24 hours: analysis of arterial blood gases showed a partial pressure of oxygen of 39 mmHg. The decision was made to place the patient on ECMO as the only viable way to achieve adequate oxygenation, and with the expectation that the pneumonia would respond to the steroids, given enough time.
The patient was taken to the cardiac catheterization laboratory, where the TandemHeart System's catheters would be used to provide a circuit for ECMO. A 25F cannula was placed into the patient's inferior vena cava (IVC) percutaneously through the left femoral vein, to provide inflow to the ECMO device. A 21F cannula was placed via the right femoral vein into the right atrium in order to provide outflow from the ECMO device to the patient. A Jostra QUADROX oxygenator (Maquet Cardiopulmonary AG; Hirrlingen, Germany) with a Bio-Medicus® pump (Medtronic, Inc.; Minneapolis, Minn) provided the ECMO. The patient was initially placed on venovenous ECMO at a flow rate of 4.5 L/min. Immediately after initiation, this intervention resulted in a dramatic improvement in gas exchange.
The patient required ECMO support for 12 days. She experienced the common complications of ECMO, including some minor bleeding from the cannula sites. After a 2-month stay, the patient was discharged from thehospital on room air with a clear chest radiograph and no apparent neurologic or other organ dysfunction. At 3 years of follow-up, she was doing well, without evident recurrence of pneumonia.
Patient 2
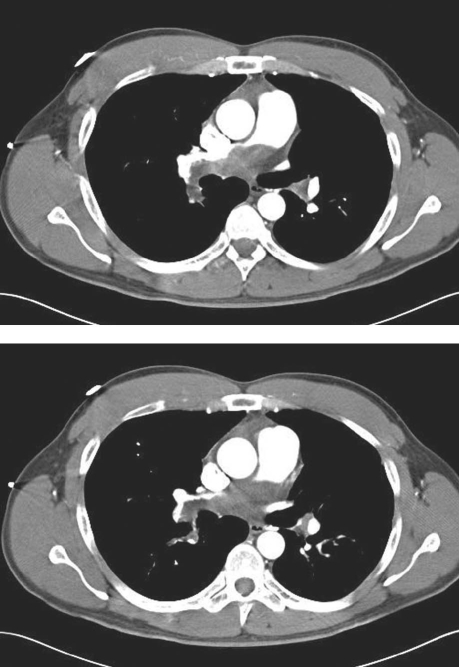
In June 2006, a 40-year-old man presented at anotherfacility with progressive dyspnea of 6 weeks' duration. He had no relevant medical history. His physical examination was unremarkable except for jugular venous distention and a split pulmonic 2nd sound. Upon auscultation, his lungs were clear. Laboratory data were noteworthy for an oxygen saturation of 90% on room air and a hemoglobin level of 17.1 g/dL. The patient's chest radiograph showed clear lung fields; however, computed tomography showed large filling defects of the proximal right and left pulmonary arteries (Fig. 3). The diagnosis was pulmonary embolism, and he was admitted to that hospital for anticoagulation with heparin. After a few days, he was discharged with instructions to take warfarin.
Fig. 3 Patient 2. Chest computed tomograms show large proximal right and left pulmonary artery filling defects, which were initially thought to reflect pulmonary emboli.
The patient soon returned to the emergency room because of syncope and worsening shortness of breath. Computed tomography showed no improvement or worsening of the pulmonary artery filling defects. The medical therapy apparently having failed, he was transferred to our institution for thromboendarterectomy. Preoperative transesophageal echocardiography was performed in order to evaluate the embolism and its consequent hemodynamic effects. The imaging showed a moderately dilated right atrium and right ventricle, and systolic pulmonary artery pressure was estimated to be at least 60 mmHg.
Thromboendarterectomy was performed in the standard fashion, apparently successfully, with use of traditional CPB. However, the patient's postprocedural weaning from CPB was complicated by severe pulmonary hypertension, right ventricular dilation, and right-heart failure of such severity as to cause cardiac output insufficiency. Pharmacologic interventions did not reduce right ventricular afterload sufficiently to enable LV filling. Therefore, ECMO support was planned. Under fluoroscopy, a 21F TandemHeart cannula was placed into the patient's left atrium, and a previously placed 28F bicaval cannula was used for the inflow—constituting a novel right atrial–oxygenator–left atrial ECMO circuit.
The ECMO circuit provided very effective systemic oxygenation for 8 days. However, the patient's pulmonary artery hypertension never improved. Histopathologic evaluation of the endarterectomy specimen revealed his pulmonary artery obstruction to be a myxoid leiomyosarcoma arising from the pulmonary artery. The application for heart–lung transplantation was denied because of the neoplasm. Consequently, the patient's family requested that support be withdrawn, and he died.
Discussion
We have reported the initial and successful use of the TandemHeart System's catheters to provide vascular access for ECMO, in 2 different clinical situations. The catheters' initial deployment was to provide conventional venovenous ECMO support in primary respiratory failure. Having facilitated effective support in that circumstance, the catheters were then used—via a novel vascular circuit—to provide ECMO for a patient who was experiencing severe pulmonary hypertension and right-heart failure. In this patient, the catheter system provided inflow to the oxygenator through a right atrial catheter and outflow to the left atrium, thereby bypassing the pulmonary vascular bed. In comparison with the usual standards of systemic oxygenation, this approach was very successful. It may even be that providing well-oxygenated blood to an intact LV, as we achieved in this case, enables a more physiologic systemic oxygen delivery than other recently used routes of ECMO support in right-heart failure (whereby oxygenated blood is delivered by catheter to the proximal aorta). In left-heart failure, we further hypothesize that the TandemHeart's catheters could be configured to provide inflow to ECMO through a left atrial catheter, with outflow through an aortic catheter. Such an approach has a theoretical advantage over the current, standard, venoarterial method of delivering ECMO in these patients: unloading the LV, and thereby in some circumstances promoting ventricular recovery.
The TandemHeart System is a relatively new, percutaneously placed device for temporary LV support.16 The system aspirates blood from the left heart via a femoral venous catheter that is introduced into the left atrium through a fluoroscopically guided transatrial septal perforation. This blood is pumped back into the proximal aorta by another catheter that is introduced through the femoral artery. The TandemHeart has been successfully used in cases of cardiogenic shock and during high-risk coronary interventions.
Patient 1 experienced chronic organizing pneumonia, an idiopathic primary inflammatory disease of the lung.17 Steroids, the primary treatment for this disorder, effect resolution in 70% to 80% of cases; however, response often takes days. By the time we had reached the diagnosis of chronic organizing pneumonia in this patient, the disease had become so severe that adequate oxygenation could not be attained, even with the use of sophisticated mechanical ventilation techniques. We reasoned that the patient's chance of a good outcome was high if we could support her via ECMO through the acute period until steroidal therapy reversed the pneumonia. We used the TandemHeart catheters to provide a venous (IVC)–oxygenator–venous (right atrial) circuit, following the protocol of recent reports of ECMO's success in respiratory failure.18,19 A large trial5 of ECMO in adults in the 1970s used a venous–oxygenator–arterial circuit, following the protocol for CPB of that time. Notably, the high airway-pressure style of mechanical ventilation that was routinely used in the 1970s was continued throughout the duration of ECMO for patients in that study. Patient survival in that series was only around 10%. Recent trials of ECMO in primary respiratory failure have used venovenous circuits, either from the femoral vein to the IVC or from the right atrium to the IVC.20 Over the past several years, protocols for ECMO have also incorporated a “lung-rest strategy,” which is now thought to be crucial in order to minimize ventilator-induced lung trauma and consequently to enable recovery from acute lung injury.20 Reports of success from these trials suggest that ECMO is a viable and effective method of support in reversible lung injury that is complicated by refractory hypoxemia.8–10 We have shown that the TandemHeart System can provide effective vascular access and conduits for ECMO in this circumstance.
Patient 2 had severe pulmonary hypertension that caused respiratory failure and right-heart decompensation after pulmonary endarterectomy. There are several prior reports of successful temporary ECMO use in severe pulmonary hypertension that had been caused by a variety of conditions, including chronic pulmonary emboli,21 progression of primary pulmonary hypertension (as a bridge to transplantation), and acute right-heart failure that is associated with heart transplantation.16 A right atrial–aortic conduit is typically established in these circumstances, either percutaneously or surgically, through which ECMO is delivered. In our patient, we used the TandemHeart catheters percutaneously to provide inflow to the oxygenator from the right atrium, as has been done, but we placed the outflow port in the left atrium—a novel position—through a transseptal puncture. Without proof, we hypothesized that returning oxygenated blood to an intact left heart could promote better oxygen delivery systemically (especially to the brain) than would the use of an outflow catheter in the aorta. On the basis of measurements of arterial and mixed venous blood gases, as well as organ function, this approach worked well to oxygenate the patient, even though his pulmonary artery disorders proved irreversible. Our experience establishes this TandemHeart catheter configuration as a viable and perhaps even superior method of providing a vascular route for ECMO support in the presence of severe, acute, reversible pulmonary outflow obstruction.
Several centers have successfully used ECMO to provide temporary support in acute, life-threatening left-heart failure.22 Various circuits have been used, but most often a right atrial catheter has provided venous inflow to the oxygenator, and an aortic catheter has been the conduit for outflow. In spite of those successes, ECMO as a stand-alone support for this indication carries with it some substantial problems: ECMO does not decompress the LV and can, in fact, increase LV afterload and wall stress. This can promote pulmonary edema, hemorrhage, and pulmonary hypertension, and hamper myocardial recovery from an acute injury.13 Intra-aortic balloon counterpulsation is therefore often used in conjunction with ECMO in this situation. There is even a report of placing a percutaneous LV assist device for this purpose.23 The TandemHeart catheters, with their ability to unload the LV via a transseptally placed left atrial inflow catheter, could possibly provide a superior circuit for ECMO in patients who are experiencing such severe, acute LV failure that systemic oxygenation is threatened. We have not yet used the TandemHeart in this situation.
The TandemHeart catheters imaginably accord different vascular routes for the delivery of ECMO, thus enabling optimal oxygen delivery in various types of cardiopulmonary failure. In cases of respiratory failure from primary lung injury, the system could provide venovenous ECMO through an IVC–right atrial conduit; in severe pulmonary vascular circumstances or right-heart failure, a right atrial–left atrial circuit; and in life-threatening left-heart failure, a left atrial–aortic route.
In conclusion, we have shown that the TandemHeart System's catheters can provide effective short-term circuits for the implementation of ECMO. Perhaps more important, the catheter system can provide novel and perhaps superior vascular routes for the delivery of ECMO in different circumstances of cardiopulmonary failure.
Footnotes
Address for reprints: James P. Herlihy, MD, 6624 Fannin St., Suite 1730, Houston, TX 77030
E-mail: jph@houstonlungdocs.com
References
- 1.Cartwright RS, Lim TP, Luft UC, Palich WE. A study of the physiological changes in the lungs during cardiopulmonary bypass. Surg Forum 1960;11:226–8. [PubMed]
- 2.Cartwright RS, Magovern GJ. Postoperative care after cardiopulmonary bypass for open heart surgery. Am Surg 1960; 26:65–72. [PubMed]
- 3.Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286(12):629–34. [DOI] [PubMed]
- 4.Frenckner B, Radell P. Respiratory failure and extracorporeal membrane oxygenation. Semin Pediatr Surg 2008;17(1):34–41. [DOI] [PubMed]
- 5.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242(20):2193–6. [DOI] [PubMed]
- 6.Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg 1997;226(4):544–66. [DOI] [PMC free article] [PubMed]
- 7.Kopp R, Henzler D, Dembinski R, Kuhlen R. Extracorporeal membrane oxygenation by acute respiratory distress syndrome [in German]. Anaesthesist 2004;53(2):168–74. [DOI] [PubMed]
- 8.Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006;6:163. [DOI] [PMC free article] [PubMed]
- 9.Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 2008;134(1):179–84. [DOI] [PubMed]
- 10.Meyer A, Struber M, Fischer S. Advances in extracorporeal ventilation. Anesthesiol Clin 2008;26(2):381–91, viii. [DOI] [PubMed]
- 11.Gregoric ID, Chandra D, Myers TJ, Scheinin SA, Loyalka P, Kar B. Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2008;27(4):466–8. [DOI] [PubMed]
- 12.Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 2001;122(1):92–102. [DOI] [PubMed]
- 13.Hoefer D, Ruttmann E, Poelzl G, Kilo J, Hoermann C, Margreiter R, et al. Outcome evaluation of the bridge-to-bridge concept in patients with cardiogenic shock. Ann Thorac Surg 2006;82(1):28–33. [DOI] [PubMed]
- 14.Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, et al. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg 2005;79(1):178–84. [DOI] [PubMed]
- 15.Doll N, Kiaii B, Borger M, Bucerius J, Kramer K, Schmitt DV, et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg 2004;77(1):151–7. [DOI] [PubMed]
- 16.Gregoric ID, Jacob LP, La Francesca S, Bruckner BA, Cohn WE, Loyalka P, et al. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge). Tex Heart Inst J 2008;35(2):125–9. [PMC free article] [PubMed]
- 17.Schlesinger C, Koss MN. The organizing pneumonias: a critical review of current concepts and treatment. Treat Respir Med 2006;5(3):193–206. [DOI] [PubMed]
- 18.Beiderlinden M, Eikermann M, Boes T, Breitfeld C, Peters J. Treatment of severe acute respiratory distress syndrome: role of extracorporeal gas exchange. Intensive Care Med 2006; 32(10):1627–31. [DOI] [PubMed]
- 19.Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest 1997;112(3):759–64. [DOI] [PubMed]
- 20.Kopp R, Dembinski R, Kuhlen R. Role of extracorporeal lung assist in the treatment of acute respiratory failure. Minerva Anestesiol 2006;72(6):587–95. [PubMed]
- 21.Ogino H, Ando M, Matsuda H, Minatoya K, Sasaki H, Nakanishi N, et al. Japanese single-center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 2006;82(2):630–6. [DOI] [PubMed]
- 22.Copeland J. Invited commentary. Ann Thorac Surg 2006;82 (1):33–4. [DOI] [PubMed]
- 23.Vlasselaers D, Desmet M, Desmet L, Meyns B, Dens J. Ventricular unloading with a miniature axial flow pump in combination with extracorporeal membrane oxygenation. Intensive Care Med 2006;32(2):329–33. [DOI] [PubMed]