Figure 7.
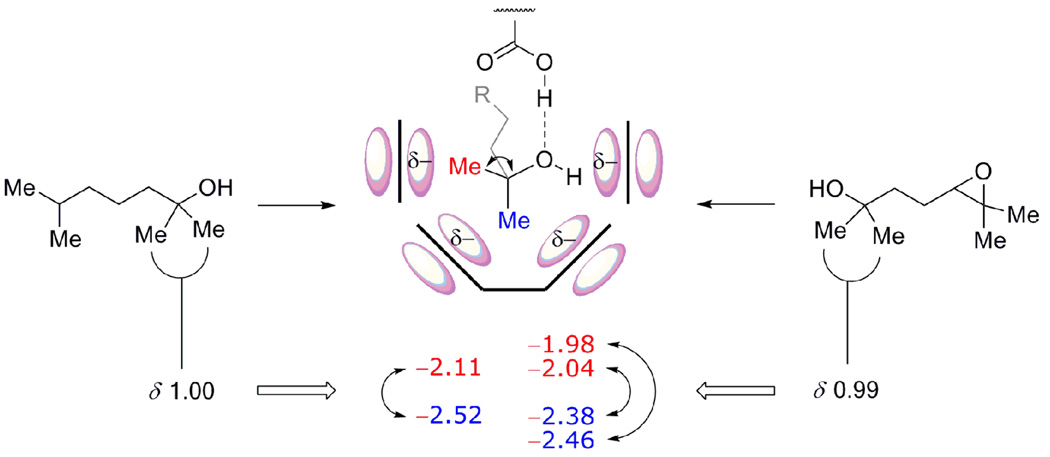
Different stereoelectronic environments experienced by the buried methyl groups of 2 and 6 bound within 1. The methyl group directed towards the cavitand wall (equatorial, in red) only experiences the anisotropic shielding of a single aromatic ring at a given time whiles the one deep in the cavity (axial, blue) experiences the effects of the four aromatics of the resorcinarene cone (NMR shifts expressed in ppm).