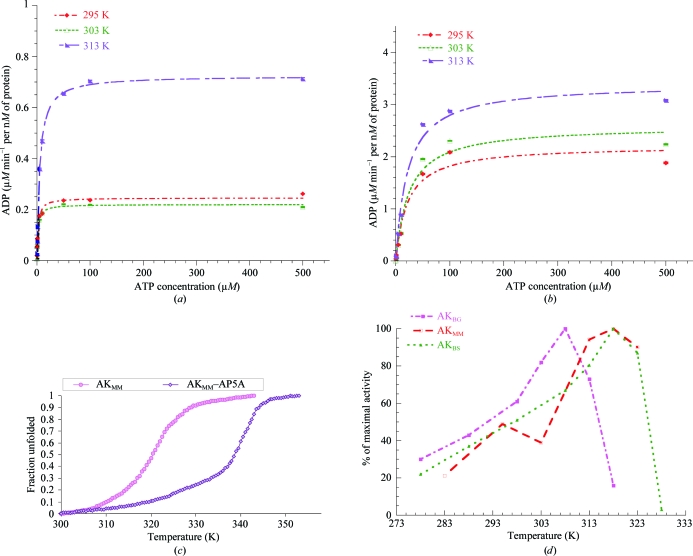
Figure 1.
(a) ATP-dependent reaction kinetics for the production of ADP by AKMM from 293 to 313 K. ATP was used as the variable substrate at concentrations of 0.1, 0.5, 1, 5, 10, 50, 100 and 500 µM at each temperature. The concentrations of AKMM (9 nM) and MgAMP (1400 µM) were kept constant. The kinetic constants determined from these experiments are summarized in Table 1 ▶. (b) AMP-dependent reaction kinetics for the production of ADP by AKMM from 293 to 313 K. AMP was used as the variable substrate at concentrations of 0.1, 0.5, 1, 5, 10, 50, 100 and 500 µM at each temperature. The concentrations of AKMM (9 nM) and MgATP (1400 µM) were kept constant. The kinetic constants determined from these experiments are summarized in Table 1 ▶. (c) Thermal denaturation midpoints (T m) of AKMM and the AKMM–AP5A complex from circular dichroism. The midpoint of the transition for unfolding of AKMM at 20 µM occurs at 321.1 K, while the midpoint of unfolding for the AKMM–AP5A complex is 340.6 K, 19.5 K higher than for the free protein. (d) Temperature profile for the activity of M. marinus and Bacillus AKs. The temperature–activity profile for AKMM has a much broader temperature range that is more similar to that of the mesophilic AK. The data for B. globisporus and B. subtilis AKs were taken from Bae & Phillips (2004 ▶).