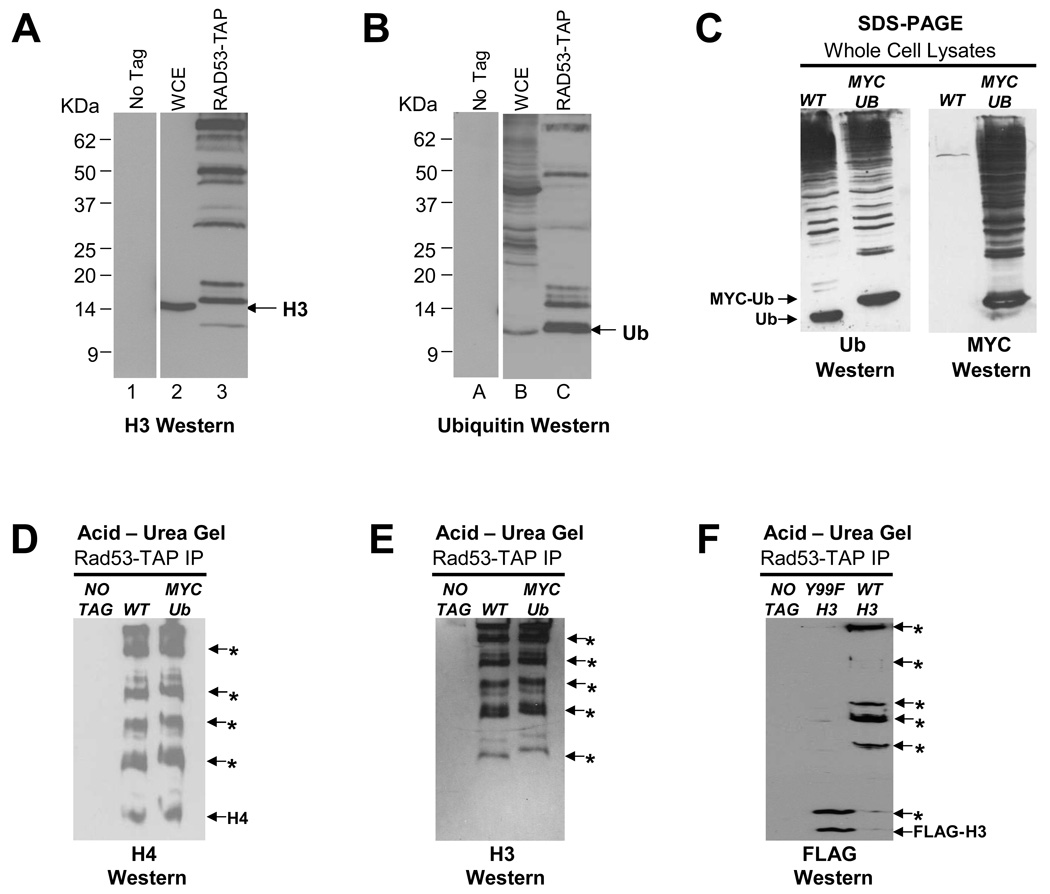
Figure 2. Histones associated with Rad53 are ubiquitylated and the tyrosine 99 residue of histone H3 is critical for its ubiquitylation.
(A) Histone H3 associated with Rad53 is extensively modified. Rad53-TAP complexes were immunoprecipitated as described in Methods and analyzed by Western blotting with H3-C antibody. The migration of bulk histone H3 in WCE is indicated by the arrow. In Rad53 immunoprecipitates, multiple bands with slower mobility than the H3 band in WCE are detected, suggesting that histone H3 associated with Rad53 is heavily modified.
(B) Histone H3 associated with Rad53 appears to be ubiquitylated. The Western blot shown above in (A) was stripped and reprobed with an ubiquitin antibody. The migration of free ubiquitin (Ub) is indicated by the arrow.
(C) Characterization of a strain in which the only source of ubiquitin is His6-MYC tagged (MYC-Ub). WCEs prepared from wild type (WT) and MYC-Ub strains were resolved by SDS-PAGE and processed for Western blotting first with a ubiquitin (Ub) antibody (Ub Western) and, after stripping, with a MYC antibody (MYC Western).
(D) Histone H4 bound to Rad53 is either multi- or poly-ubiquitylated. Histone H4 present in Rad53-TAP immunoprecipitates was analyzed by acetic acid-urea (AU) gel electrophoresis followed by Western blotting with H4 antibodies. The mobility of unmodified histone H4 is indicated by “H4” while the putative ubiquitylated histone H4 species carrying increasing numbers of ubiquitin moieties are indicated by asterisks. The mobility of H4-MYC-Ub bands is slightly retarded in the lane containing Rad53-TAP immunoprecipitates from the MYC-Ub strain compared to those derived from the WT strain.
(E) Histone H3 bound to Rad53 is also either multi- or poly-ubiquitylated. Indicated samples were processed as described in (D) except that Western blotting was carried out to detect histone H3. Note that in this experiment all histone H3 appears to be modified.
(F) Tyrosine 99 residue of histone H3 is required for the efficient ubiquitylation of this histone. Strains carrying FLAG-tagged endogenous genes corresponding to either the wild type (WT) or Y99F mutant histone H3 were processed as described in (D) except that Western blotting was carried out using FLAG antibodies. Note the absence of high molecular mass FLAG-H3 species in the Y99F mutant.