Abstract
5-HT2-type serotonin receptors (5-HT2Rs) are widely expressed throughout the brain and mediate many of the modulatory effects of serotonin. It has been thought that postsynaptic 5-HT2Rs act primarily by depolarizing neurons and thereby increasing their excitability. However, it is also known that 5-HT2Rs are coupled to Gq/11-type G-proteins and that some other types of Gq/11-coupled receptors can regulate synapses by evoking endocannabinoid release and activating presynaptic cannabinoid-type 1 receptors (CB1Rs). Here, we examine whether activation of 5-HT2Rs can regulate synapses through such a mechanism by studying excitatory synapses onto cells in the inferior olive (IO). These cells express 5-HT2Rs on their soma and dendrites, and the IO receives extensive serotonergic input. We find that the excitatory synaptic inputs onto IO cells are strongly suppressed by serotonin receptor agonists as well as release of endogenous serotonin. Both 5-HT2Rs and 5-HT1BRs contribute to this modulation by decreasing the probability of glutamate release from presynaptic boutons. The suppression by 5-HT2Rs is of particular interest because it is prevented by CB1R antagonists, and 5-HT2Rs are thought to be located only postsynaptically on IO cells. Our results indicate that serotonin activates 5-HT2Rs on IO neurons, thereby releasing endocannabinoids that act retrogradely to suppress glutamate release by activating presynaptic CB1Rs. These findings establish a link between serotonin signaling and endocannabinoid signaling. Based on the extensive distribution of 5-HT2Rs and CB1Rs, it seems likely that this mechanism could mediate many of the actions of 5-HT2Rs throughout the brain.
Keywords: synaptic plasticity, serotonin, endocannabinoid, inferior olive, retrograde inhibition, 5-HT2 receptor
Introduction
Activation of postsynaptic 5-HT2-type serotonin receptors (5-HT2Rs) is known to alter cell excitability by depolarizing cells (Barnes and Sharp, 1999; Leysen, 2004). 5-HT2Rs are known to couple to Gq/11-type G-proteins (Barnes and Sharp, 1999; Hoyer et al., 2002; Leysen, 2004; Parrish and Nichols, 2006), and activation of some other types of Gq/11-coupled receptors, such as metabotropic glutamate mGluR1/5 and muscarinic acetylcholine receptors, can promote endocannabinoid release from the dendrites of cells (Maejima et al., 2001; Varma et al., 2001; Kim et al., 2002; Chevaleyre et al., 2006; Oliet et al., 2007). Endocannabinoids can cross the synaptic cleft and bind to presynaptic cannabinoid-type 1 receptors (CB1Rs), which reduce the probability of transmitter release (Chevaleyre et al., 2006). Here we test the hypothesis that activation of 5-HT2Rs can also regulate synapses by promoting endocannabinoid release.
The inferior olive (IO) is well suited to testing this possibility. 5-HT2ARs are highly expressed on the dendrites and soma of neurons within the IO (Fay and Kubin, 2000; Fonseca et al., 2001), and the IO receives a dense projection from serotonergic brainstem nuclei (Takeuchi and Sano, 1983; Bishop and Ho, 1986). Binding studies indicate that cannabinoid receptors are present in the IO (Mailleux and Vanderhaeghen, 1992), and phospholipase Cβ4 (PLCβ4), which is known to be important for Gq/11-dependent endocannabinoid release, is expressed in the dendrites of IO cells (Nakamura et al., 2004). Finally, it is possible to cut a slice containing the IO and brainstem nuclei that provide serotonergic inputs, raising the possibility of examining the effects of endogenous serotonin on synaptic transmission.
For many regions of the IO, such as the dorsal principal olive (dPO) studied here, the mesodiencephalic regions (MDRs) are the primary source of excitatory inputs. More specifically, the dPO receives excitatory inputs from MDRs such as the parvocellular red nucleus, the nucleus of Darkschewitsch, and the nucleus of the optic tract (Swenson and Castro, 1983a,b; Azizi and Woodward, 1987; de Zeeuw et al., 1990; De Zeeuw et al., 1998). The properties and plasticity of these synapses have not been determined.
Here we examine excitatory synapses onto dPO cells in rat brain slices. We find that serotonin strongly suppresses EPSCs both by activating presynaptic 5-HT1BRs and by activating postsynaptic 5-HT2Rs. The activation of 5-HT2Rs evokes endocannabinoid release from dPO neurons, which binds to presynaptic CB1Rs to decrease the probability of release. Furthermore, we find that stimulation of serotonergic brainstem nuclei within our slices can release sufficient serotonin to activate 5-HT2Rs and result in endocannabinoid-mediated suppression of excitatory synapses onto dPO cells. These findings establish a link between serotonin and cannabinoid signaling systems.
Materials and Methods
Tissue preparation.
Sprague Dawley rats were deeply anesthetized with ketamine/xylazine, placed in an ice bath, and transcardially perfused with an ice-cold sucrose solution consisting of the following (in mm): 82.7 NaCl, 23.8 NaHCO3, 71.2 sucrose, 23.7 glucose, 2.4 KCl, 1.4 NaH2PO4, 6.8 MgCl2, and 0.5 CaCl2. Brains were quickly removed and placed in ice-cold sucrose solution. The brainstem was isolated and placed on its dorsal surface on an agar block. The agar block was cut so that the brainstem would lean forward ∼30° toward the blade when placed upright on the slicing pedestal. Sections, 250 μm thick, containing the inferior olive were cut and transferred to the recording solution that consisted of the following (in mm): 125 NaCl, 26 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, and 2 CaCl2. Slices were incubated for 45 min at 32°C and then allowed to cool to room temperature. All solutions were bubbled with 95% O2/5% CO2. Experiments were performed at 34–35°C using an in-line heater (Warner Instruments). Recording solution flow rates were 3.5–4 ml/min. Postnatal day 10 (P10) to P15 rats were used for experiments. In older animals, active conductances precluded the accurate quantification of EPSCs. Based on electron microscopic examination of the medial accessory olive, it is likely that gap junctional coupling within the dPO first begins to appear at P10 and is relatively mature by P15 (Bourrat and Sotelo, 1983). The active conductances we record along with EPSCs begin to develop during this period and become prominent by P15. We found that the active conductances do not result from gap junctional currents because they were not prevented by application of the gap junction blocker mefloquine. Preliminary experiments in older animals in which we observed active components associated with the EPSCs showed qualitatively similar results. All procedures involving animals were approved by the Harvard Medical Area Standing Committee on Animals.
Electrophysiology.
Whole-cell voltage-clamp recordings were obtained from dPO neurons using a Multiclamp 700A (Molecular Devices). Recording pipettes had a resistance of 1.8–3.4 MΩ when filled with internal recording solution. Cells were held at −70 mV. The internal recording solution for voltage-clamp experiments consisted of the following (in mm): 145 Cs methane sulfonate, 15 HEPES, 0.2 EGTA, 1 MgCl2, 5 tetraethylammonium-Cl, 2 Mg-ATP, 0.3 Na-GTP, 10 phosphocreatine (tris), and 2 N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium (QX-314) (adjusted to ∼315 mOsm, pH 7.3). In some experiments C14H22N2O was inadvertently used instead of QX-314. Data were similar in both conditions. The internal recording solution for current-clamp experiments consisted of the following (in mm): 114 K methane sulfonate, 6 NaCl, 10 HEPES, 0.5 EGTA, 2 MgSO4, 0.16 CaCl2, 4 Na-ATP, 0.4 Na-GTP, and 14 phosphocreatine (Tris) (adjusted to ∼315 mOsm, pH 7.3). EPSCs were evoked in the presence of picrotoxin (50 μm) to block GABAARs, CGP 55845 [(2S)-3-[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)-phosphinic acid] (2 μm) to block GABABRs, and strychnine (1 μm) to block glycine receptors. Experiments involving pressure-applied glutamate were done in the presence of TTX (500 nm) to block voltage-gated sodium channels, 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2 carboxamide hydrochloride (YM 298198) (5 μm) to block mGluR1 receptors, and 2-methyl-6-(phenylethynyl)-pyridine (MPEP) (5 μm) to block mGluR5 receptors. Two of the four experiments involving pressure-applied glutamate were recorded in the presence of 3-((R)-2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid (R-CPP) (5 μm) to block NMDA receptors. For stimulation of serotonergic brainstem nuclei, stainless steel Teflon-coated bipolar electrodes (0.005 inch wire; A-M Systems) were placed as in Figure 5A. All experiments involving stimulation of endogenous serotonin release were done in the presence of YM 298198 (5 μm) and MPEP (5 μm) to prevent inadvertent activation of PLC through mGluR1 and mGluR5. Numerical values used in the text and in bar graphs were based on the average amplitudes of the first two test stimuli after the conditioning train.
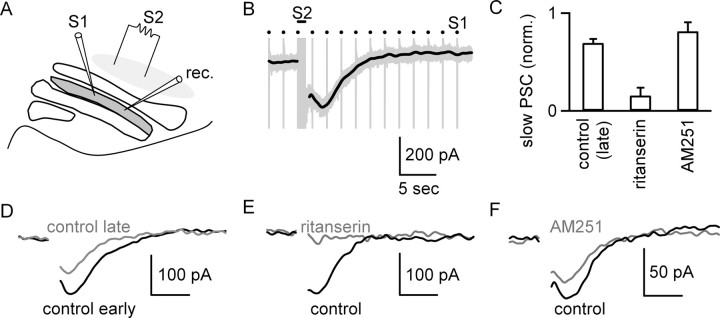
Figure 5.
Stimulation of serotonergic brainstem nuclei produces a slow postsynaptic current in IO cells. A, A schematic illustrates the experimental configuration used to assess the effects of activation of serotonergic brainstem nuclei that project to the IO. Electrode S1 was used to stimulate excitatory inputs, and electrode S2 was used to activate serotonergic fibers. B, Raw trace (gray) illustrates the response to stimulation of EPSCs at 0.5 Hz with S1 and stimulation of serotonergic brainstem nuclei with S2 (bar, 50 stimuli at 50 Hz), which produced a slow PSC. The slow PSC could be studied in isolation by removing the stimulus artifacts and low-pass filtering at 1 Hz (black trace). C, Bar graph summarizes the amplitude of the slow PSC measured in trials separated by 9 min, with the first trial in control conditions and the second trial, in either control conditions (n = 4 cells) or the presence of either ritanserin (n = 8 cells) or AM251 (n = 4 cells). D, Example slow PSCs recorded in early (black) and late (gray) control conditions. E, Example slow PSCs recorded before (black) and after (gray) the blockade of 5-HT2Rs with ritanserin. F, Example slow PSCs recorded in the presence of NAS-181 before (black) and after (gray) application of AM251. Data are means ± SEM.
Axons from the MDR do not form well defined fiber tracts as they enter the IO, and it is impractical to cut slices that include the IO and the MDR. Because IO projection neurons are not thought to give rise to excitatory local collaterals (Desclin and Colin, 1980; Foster and Peterson, 1986; De Zeeuw et al., 1998), we stimulated EPSCs by placing pairs of glass pipettes into the neuropil of the dPO. For experiments involving agonist application, pairs of EPSCs were evoked with an interstimulus interval of 20 ms at 0.1 Hz. Systematic increases in the stimulus intensity were accompanied by gradual increases in the size of the evoked EPSC, suggesting that individual synaptic currents were small. Stimulus strength was adjusted to activate EPSCs of >400 pA, which consisted of many synaptic inputs and provided a stable response. In experiments in which currents were evoked by pressure application of glutamate (1 mm, 20 ms pulses at 0.1 Hz), the pressure (10–20 psi) was adjusted to evoke currents of <400 pA. Electrodes 2.5 μm in tip diameter were placed ∼10 μm into the slice at a position 10–40 μm from the cell body. Only minor movements were observed. Vehicles were balanced across solutions during consecutive application of agonists followed by antagonists. Solution tubing was changed after application of N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) R-(+)-(2,3-dihydro-5-methyl-3-[(4-morpholinyl)methyl]pyrol[1,2,3-de]1,4-benzoxazin-6-yl)-(1-naphthalenyl) methanone (WIN 55,212-2; WIN), or ritanserin. DMSO concentrations were < 0.001%. AM251, CGP 55845, (2R)-2-[[[3-(4-morpholinylmethyl)-2H-1-benzopyran-8-yl]oxy]methyl]morpholine dimethanesulfonate (NAS-181), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), R-CPP, ritanserin, (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2), MPEP, YM 298198, and WIN 55,212-2 were purchased from Tocris Cookson. All other chemicals were purchased from Sigma.
Data acquisition and analysis.
Recordings were filtered at 3 kHz and sampled at 20 kHz with a 16-bit analog-to-digital converter (ITC-18; InstruTech). All analysis was performed using custom macros written in Igor Pro (Wavemetrics). Statistical significance was determined with t tests and ANOVAs. Tukey's tests were used for post hoc analysis. Statistical significance was defined as p < 0.05. All data are expressed as means ± SEM.
Results
EPSCs recorded from IO cells are suppressed by serotonin
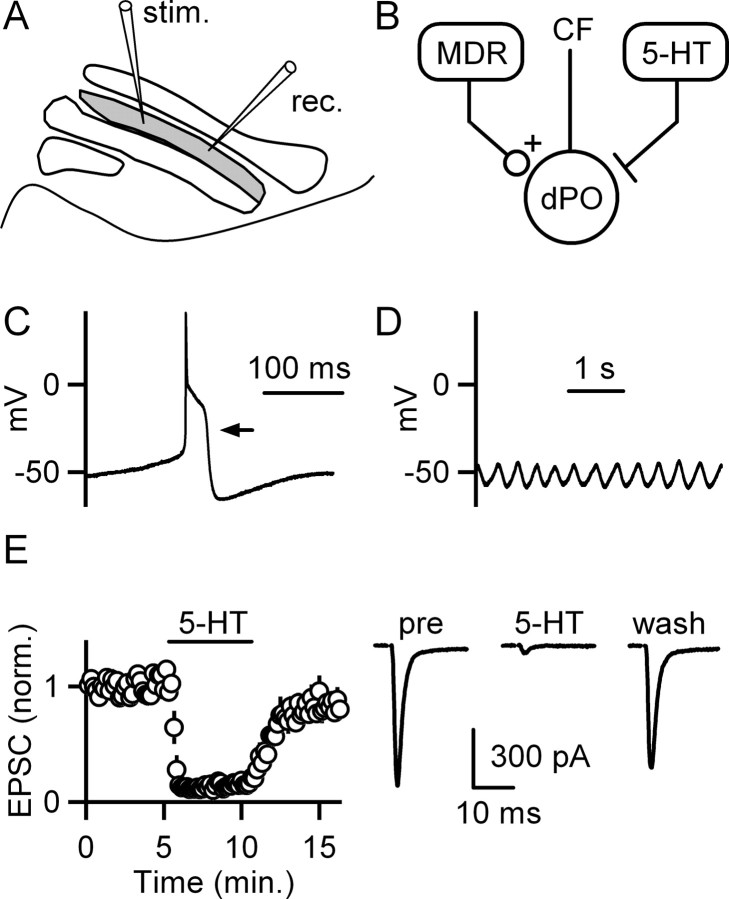
Whole-cell recordings were obtained from neurons within the dPO region of the IO in brainstem slices (Fig. 1A, gray region). The dPO is readily identified within our transverse slice preparation using the white matter outlining each subnucleus as a reference. Although a few inhibitory interneurons are present within some IO subnuclei, they are not commonly found in the rat dPO, which consists almost exclusively of large excitatory projection neurons that give rise to the climbing fibers that innervate Purkinje cells (Fredette et al., 1992; De Zeeuw et al., 1998). Recordings from these neurons within the dPO exhibited properties stereotypical of IO projection neurons. Each action potential was associated with a high-threshold calcium spike (Fig. 1C, arrow) when the initial membrane potential was near −55 mV (Llinas and Yarom, 1981a,b) and robust subthreshold oscillations were apparent (Fig. 1D). Thus, our recordings are from IO projection neurons within the dPO.
Figure 1.
Excitatory synapses onto inferior olive neurons are suppressed by serotonin. A, A schematic of the slice preparation illustrates recording (rec.) and stimulation (stim.) sites within the dPO (gray) of the IO. B, A simplified circuit shows that neurons in MDRs provide excitatory synapses to neurons in the dPO. Brainstem nuclei provide a strong serotonergic (5-HT) input to the IO. Cells in the IO provide climbing fiber (CF) synapses whose primary target is Purkinje cells in the cerebellar cortex. C, Example recordings from dPO cells using a potassium-based pipette solution show a spontaneous action potential (C) and spontaneous subthreshold membrane potential oscillations (D). E, The effects of bath-applied 5-HT (10 μm) on EPSCs are illustrated by the time courses of EPSC amplitudes (left; n = 5 cells; means ± SEM) and by example traces from a representative experiment (right).
Anatomical studies suggest that the dPO receives excitatory synapses from the MDR (Fig. 1B). Synaptic inputs were stimulated with an electrode placed in the neuropil of the dPO (Fig. 1A). The EPSCs we recorded from dPO cells had consistent properties. EPSCs were recorded from dPO cells by stimulating in the presence of blockers of inhibitory neurotransmission and could be blocked by the AMPA receptor antagonist NBQX (5 μm) and the NMDA receptor antagonist R-CPP (5 μm) (data not shown).
Neuromodulatory fibers from serotonergic brainstem nuclei also provide a powerful input to the IO (Fig. 1B) (Takeuchi and Sano, 1983; Bishop and Ho, 1986). Previous studies have shown that serotonin can depolarize neurons within the IO (Placantonakis et al., 2000), but it was not known whether serotonin regulates synaptic inputs to these cells. We found that bath application of 5-HT (10 μm) suppressed EPSCs recorded from dPO cells to 14 ± 3% (n = 5 cells) of control (Fig. 1E).
IO cells are known to strongly express 5-HT2ARs, which are coupled to the G-protein Gq/11 (Fay and Kubin, 2000; Fonseca et al., 2001). We therefore tested whether 5-HT2Rs were involved in the suppression of excitatory synapses. Because selective 5-HT2AR agonists are not available (Leysen, 2004), we attempted to attain selective activation of 5-HT2ARs by using a low concentration of the high-affinity synthetic 5-HT2AR agonist TCB-2. Although TCB-2 is reported to activate 5-HT2ARs, it is not known how selective this compound is for 5-HT2ARs. TCB-2 (1 μm) mimicked the effect of serotonin and reduced EPSCs to 15 ± 1% (n = 12 cells) of control (Fig. 2A–C). The effect of TCB-2 on EPSCs was only partially reversed by the 5-HT2R antagonist ritanserin (4 μm) (43 ± 6% of baseline; n = 4 cells) (Fig. 2A). This indicated that the suppression of EPSCs by TCB-2 involves another 5-HTR in addition to 5-HT2Rs. Previous studies at other synapses have found that activation of presynaptic 5-HT1BRs can suppress release from presynaptic boutons (Sari, 2004; Seeburg et al., 2004). We examined the role of 5-HT1BRs in modulating EPSCs onto dPO neurons by applying TCB-2, followed by coapplying TCB-2 and the 5-HT1BR antagonist NAS-181 (1 μm). NAS-181 also partially reversed the effect of TCB-2 on EPSCs (34 ± 3% of baseline; n = 4 cells) (Fig. 2B). Despite the partial recovery of TCB-2-mediated suppression by ritanserin or NAS-181 alone, coapplication of ritanserin and NAS-181 completely reversed the effect of TCB-2 (105 ± 13% of baseline; n = 4 cells) (Fig. 2C). Moreover, in the presence of NAS-181, the effect of TCB-2 was completely reversed by ritanserin (108 ± 3% of baseline; n = 4 cells) (Fig. 2D). These findings indicate that activation of 5-HT2Rs and 5-HT1BRs accounts for the EPSC suppression by TCB-2.
Figure 2.
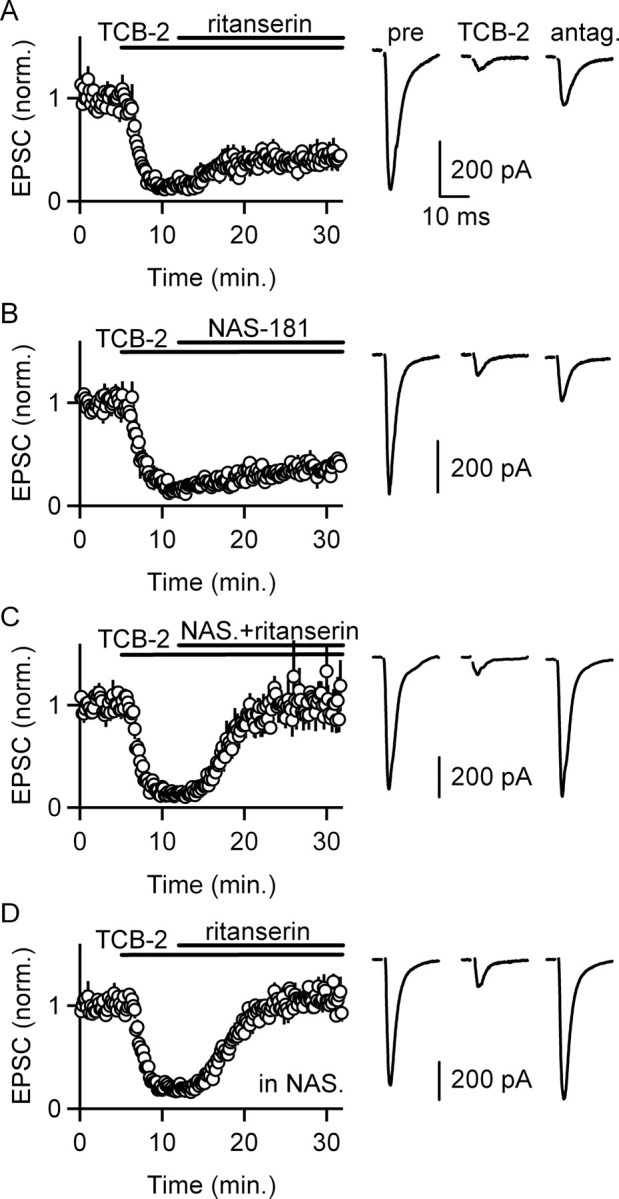
Activation of 5-HT2Rs and 5-HT1BRs suppresses EPSCs onto IO cells. A–D, Selective 5-HTR antagonists were used to characterize the receptors mediating the inhibition of EPSCs by the 5-HTR agonist TCB-2 (1 μm). Time courses of EPSC amplitudes (left column; n = 4 cells per condition) and example traces are shown (right column). The 5-HT2R antagonist ritanserin (4 μm) partially reversed the suppression of transmission (A), as did the 5-HT1BR antagonist NAS-181 (1 μm; B). C, Coapplication of ritanserin and NAS-181 reversed the suppression by TCB-2. D, In the presence of NAS-181, ritanserin completely reversed the suppression. Data are means ± SEM.
We determined whether activation of 5-HT2Rs and 5-HT1BRs modulates transmission through a presynaptic mechanism by measuring the paired-pulse ratio. Activation of 5-HT2Rs by applying TCB-2 in the presence of NAS-181 increased the paired-pulse ratio (1.43 ± 0.19 to 2.00 ± 0.22; n = 6 cells; paired t test, p < 0.05) (Fig. 3A). This suggests that 5-HT2R activation suppresses EPSCs through a presynaptic mechanism, despite the observation that 5-HT2ARs are prominently expressed postsynaptically on IO cells (Fay and Kubin, 2000; Fonseca et al., 2001). Activation of 5-HT1BRs by applying TCB-2 in the presence of ritanserin increased the paired-pulse ratio (1.42 ± 0.13 to 2.11 ± 0.15; n = 5 cells; paired t test, p < 0.05) (Fig. 3B). This suggests that 5-HT1BR activation suppresses EPSCs through a presynaptic mechanism.
Figure 3.
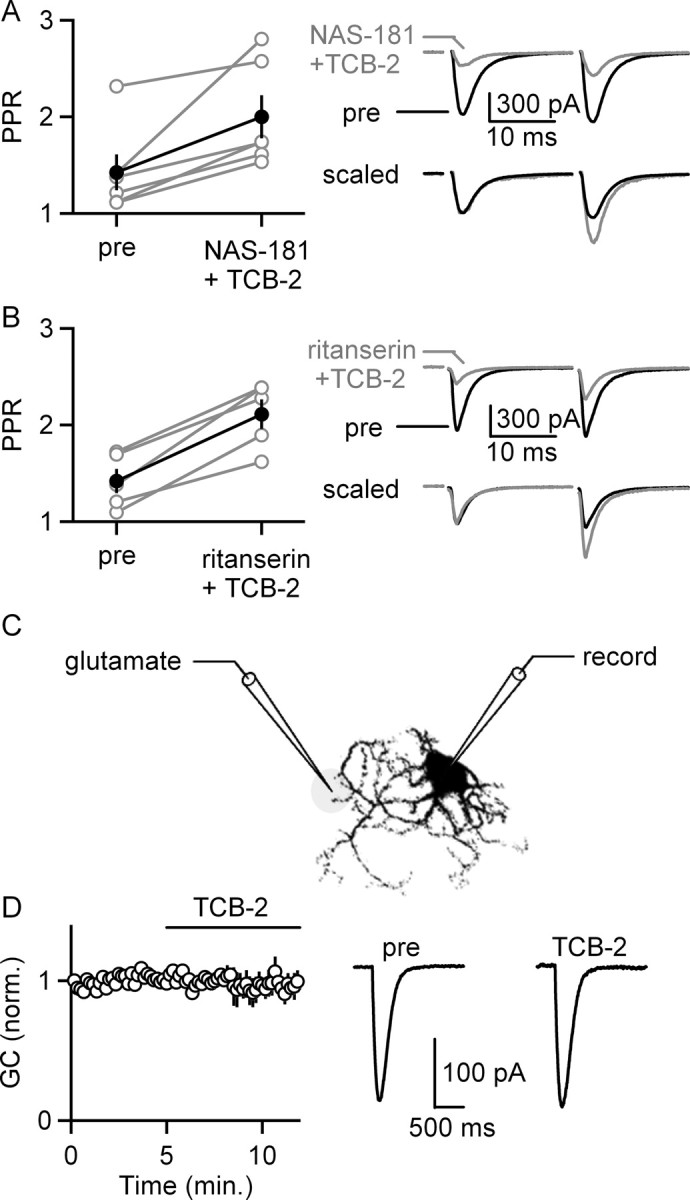
5-HT2R and 5-HT1BR activation presynaptically suppresses EPSCs onto IO cells. A, The selective activation of 5-HT2Rs by coapplying TCB-2 and NAS-181 increased the paired-pulse ratio (PPR). A summary plot shows the PPR for each experiment (open circles, left; n = 6 cells) and the average ± SEM PPR (filled circles, left). Traces are shown before and during bath application of TCB-2 (right). B, The selective activation of 5-HT1BRs by coapplying TCB-2 and ritanserin increased the paired-pulse ratio. A summary plot shows PPR for each experiment (open circles, left; n = 5 cells) and the average ± SEM PPR (filled circles, left). Traces are shown before and during bath application of TCB-2 (right). C, D, Pressure-applied glutamate was used to determine whether activation of 5-HT2Rs and/or 5-HT1BRs suppresses glutamatergic currents (GC) postsynaptically. C, A schematic illustrates the configuration used to apply glutamate onto an IO cell. D, Glutamate-evoked currents plotted as a function of time before and during TCB-2 application (left; n = 4 cells) and example traces from such an experiment are shown (right). Data are means ± SEM.
We assessed whether activation of 5-HT2Rs and 5-HT1BRs alters the response of postsynaptic glutamate receptors. Miniature EPSCs were found to be poorly suited to detect decreases in glutamate receptor sensitivity because they are small and difficult to discriminate from the noise. We therefore examined the response to pressure-applied glutamate in the presence of TTX as well as antagonists of mGluR1 and mGluR5 (Fig. 3C,D). Bath application of TCB-2, which activates both 5-HT2Rs and 5-HT1BRs, did not alter glutamate-evoked currents (98 ± 5% of control; n = 4 cells) (Fig. 3D). This suggests that alterations in postsynaptic receptor sensitivity are unlikely to contribute to synaptic suppression after 5-HT2R and 5-HT1BR activation.
5-HT2R activation suppresses EPSCs through endocannabinoid release
We next examined the mechanism of the presynaptic suppression of transmitter release produced by 5-HT2R activation. We tested the hypothesis that activation of postsynaptic 5-HT2Rs evokes endocannabinoid release from IO neurons that acts retrogradely to inhibit transmission through a presynaptic mechanism. We therefore determined whether CB1Rs are present on the presynaptic boutons of excitatory inputs. Bath application of the CB1R agonist WIN (2 μm) strongly attenuated EPSCs (8 ± 1% of control), and this suppression was reversed by the CB1R antagonist AM251 (111 ± 22% of control; n = 5 cells; 4 μm) (Fig. 4A). WIN also increased the paired-pulse ratio (1.47 ± 0.07 to 1.86 ± 0.07; n = 5 cells; one-factor ANOVA, p < 0.01), and AM251 reversed this effect (1.86 ± 0.07 to 1.42 ± 0.05) (Fig. 4B). This suggests that activation of CB1Rs located on presynaptic excitatory terminals can suppress the release of glutamate onto dPO cells.
Figure 4.
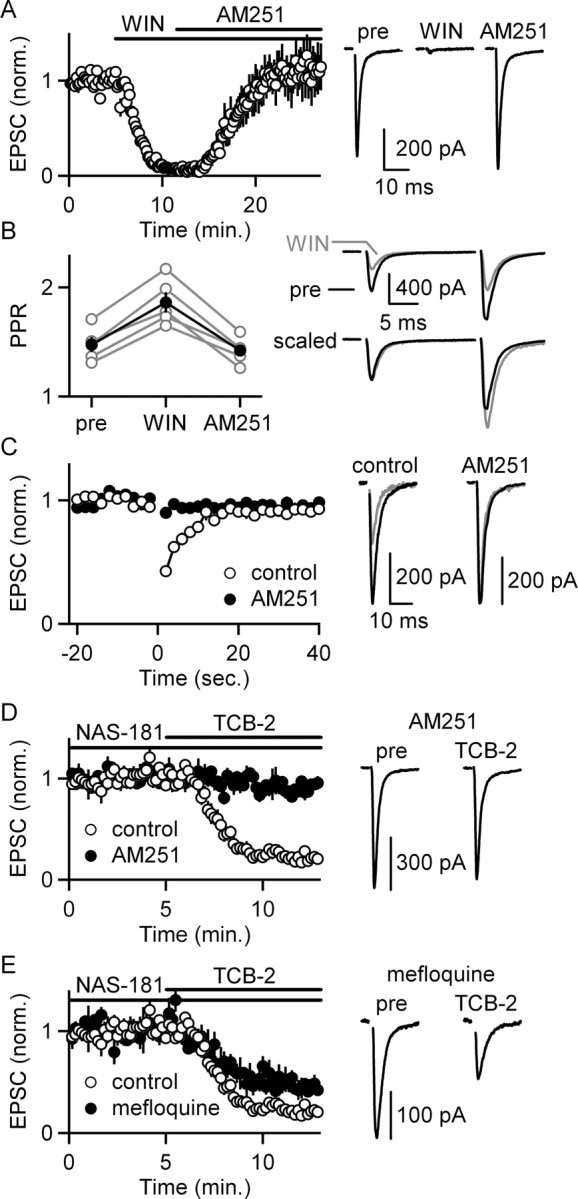
5-HT2R activation suppresses excitatory synapses onto IO cells by liberating endocannabinoids. A, Bath application of the CB1R agonist WIN (2 μm) suppresses EPSCs recorded from dPO cells, and the CB1R antagonist AM251 (4 μm) reverses this suppression. A summary of the time course of EPSC amplitudes (left; n = 5 cells) and traces from a representative experiment (right) are shown. B, WIN also increased paired-pulse ratio (PPR), and AM251 reversed this increase in PPR. A summary plot shows the PPR for each experiment (open circles, left) and the average ± SEM PPR (filled circles, left) compiled from data in A. Traces from a representative experiment are shown before (black) and during (gray) WIN application (right). C, Depolarizing dPO cells from −70 to 0 mV for 3 s (at t = 0) suppresses EPSCs for many seconds (open circles). This suppression is blocked by bath application of the CB1R antagonist AM251 (2 μm) (filled circles, left; n = 5 cells). Traces from a representative experiment are shown with the EPSCs measured before (black) and after (gray) depolarization in control conditions and in the presence of AM251. D, Blockade of CB1Rs with AM251 prevents the suppression of synaptic strength after 5-HT2R activation by applying TCB-2 in the presence of NAS-181 (left, filled circles; n = 4 cells). Example traces are shown (right). E, Blockade of gap junctions with mefloquine does not prevent the suppression of synaptic strength after 5-HT2R activation by applying TCB-2 in the presence of NAS-181 (left; n = 4 cells). Example traces are shown (right). Summaries of experiments conducted in control conditions are included for comparison (D, E, left, open circles). Example traces are shown (right). Data are means ± SEM.
Depolarization of some types of neurons evokes endocannabinoid release and leads to suppression of afferent synapses containing CB1Rs (Chevaleyre et al., 2006). We assessed whether dPO cells can release endocannabinoids by depolarizing dPO cells for 3 s. This suppressed EPSCs for many seconds, and the CB1R antagonist AM251 (2 μm) prevented this suppression (n = 5 cells) (Fig. 4C). Thus, dPO cells can release endocannabinoids from their dendrites and control the strength of their excitatory synapses.
We then tested whether CB1Rs mediate the suppression by 5-HT2Rs. The suppression of EPSCs by activation of 5-HT2Rs was prevented by including AM251 (2 μm) in the bath (91 ± 3% of control; n = 4 cells) (Fig. 4D). This suggests that activation of postsynaptic 5-HT2Rs suppresses the release of neurotransmitter and requires the activation of CB1Rs.
We also found that the prominent gap junctions between cells in the IO are not required for the suppression. The gap junction blocker mefloquine (25 μm for >1 h presoak) did not prevent the suppression mediated by 5-HT2Rs. Although mefloquine slightly reduced the extent of the suppression (45 ± 5% of baseline; n = 4 cells) (Fig. 4E) compared with control (20 ± 4%of baseline; n = 4 cells), this reduction in the extent of suppression might reflect the numerous nonspecific effects of mefloquine (Coker et al., 2000; Gribble et al., 2000; Kang et al., 2001; Cruikshank et al., 2004; Caridha et al., 2008).
Together, these findings suggest that activation of 5-HT2Rs evokes endocannabinoid release from IO cells, which acts on presynaptic CB1 receptors to reduce the probability of neurotransmitter release.
Stimulation of serotonergic brainstem nuclei
The IO receives a dense projection of serotonergic axons from brainstem nuclei, including the nucleus reticularis paragigantocellularis, which is located in close proximity to the IO (Bishop and Ho, 1986) and is contained within our brain slices. We tested whether stimulation of neurons within serotonergic brainstem nuclei is sufficient to activate serotonin receptors on IO cells within our slice preparation (Fig. 5A).
The in vivo firing properties of serotonergic neurons in the nucleus reticularis paragigantocellularis are not known. We therefore activated serotonergic brainstem nuclei with a stimulus train (50 stimuli at 50 Hz) to evoke large reproducible effects that were amenable to pharmacological analysis. In these experiments, excitatory inputs were activated with test stimuli at 0.5 Hz (Fig. 5B). Activation of serotonergic inputs had two effects: it induced an inward current in IO cells (Fig. 5), and it suppressed EPSCs (Fig. 6).
Figure 6.
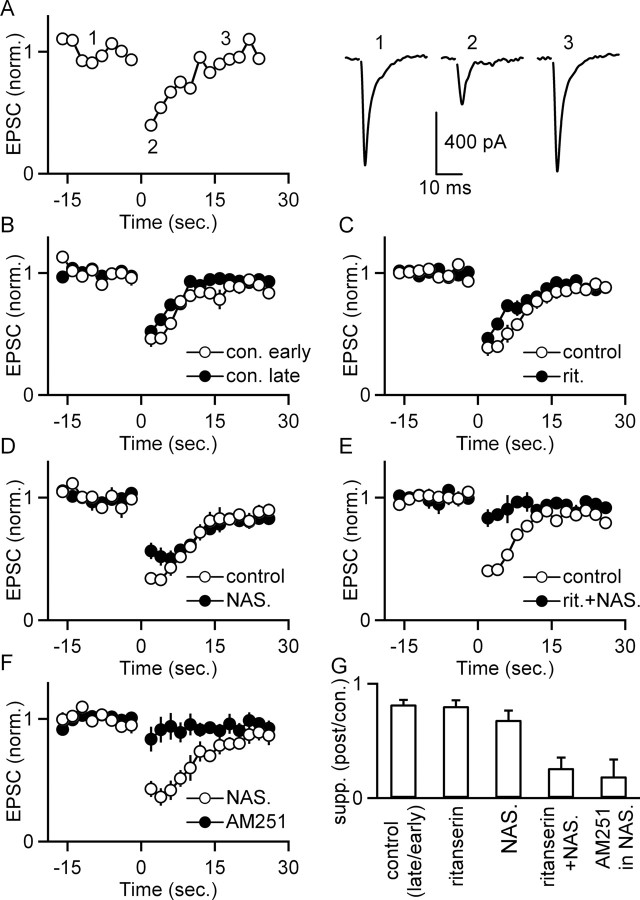
Stimulation of serotonergic brainstem nuclei suppresses EPSCs onto IO cells. The effect of activation of serotonergic inputs (50 stimuli at 50 Hz) on EPSCs was assessed using the experimental configuration of Figure 5A. A, A representative experiment shows EPSC amplitudes as a function of time (left) recorded before and after stimulation at t = 0 of brainstem serotonergic nuclei. EPSCs recorded at the indicated times are displayed (right). In B–F, experiments were conducted in which eight trials such as these were repeated at 3 min intervals, and the amplitudes of EPSCs for the first two trials (open circles) and last three trials (filled circles) were compared. In B, the entire experiment was performed in control conditions. In C–F, drugs were bath applied after the second trial and remained present for the duration of the experiment. The antagonists applied were the 5-HT2R antagonist ritanserin (C), the 5-HT1BR antagonist NAS-181 (D), ritanserin and NAS-181 (E), and the CB1R antagonist AM251 (F). G, The extent of the suppression for B–F is summarized by dividing the suppression observed in the last three trains by the suppression observed in the first two trains for each condition (n = 4 cells for each condition; data are means ± SEM).
The slow postsynaptic current (PSC) produced by activation of serotonergic brainstem nuclei was 100 ± 14 pA (n = 16 cells) and persisted for ∼10 s. Previous studies have shown that 5-HT2R agonists depolarize IO cells (Placantonakis et al., 2000), which corresponds to an inward current in voltage clamp. We therefore tested whether the slow PSC is mediated by 5-HT2Rs. Trains were delivered to serotonergic brainstem nuclei every 3 min, and this was repeated for eight trials. The slow PSCs recorded in response to the second and fifth trains were compared (Fig. 5C–F). The magnitude of the slow PSC diminished slightly over time within control conditions (69 ± 4% of early response; n = 4 cells) (Fig. 5C,D). Bath application of ritanserin immediately after the second train virtually eliminated the slow PSC (16 ± 8% of control; n = 8 cells) (Fig. 5C,E), and the reduction was significant relative to control (one-factor ANOVA, p < 0.01). This indicates that activation of serotonergic brainstem nuclei releases serotonin that activates 5-HT2Rs on IO cells. The bath application of AM251 had no effect on the magnitude of the inward current relative to control (81 ± 9% of control; n = 4 cells; one-factor ANOVA, NS) (Fig. 5C,F), indicating that AM251 does not prevent the activation of 5-HT2Rs by serotonin.
In these experiments, we also tested whether release of endogenous serotonin suppresses EPSCs onto IO cells. Stimulation of serotonergic brainstem nuclei (50 stimuli at 50 Hz) suppressed EPSCs for ∼15 s (40 ± 2% of control; n = 16 cells) (Fig. 6A–E). The average suppression in response to the first two trains was compared with the average suppression of the last three trains (Fig. 5B–G). Trials were repeated every 3 min to prevent rundown, which was small in control conditions (suppression late was 82 ± 4% of suppression early; n = 4 cells) (Fig. 6B,G). Blockade of 5-HT2Rs with ritanserin alone did not significantly affect the magnitude of the suppression (80 ± 5% of control suppression; n = 4 cells; one-factor ANOVA, NS) (Fig. 6C,G). Blockade of 5-HT1BRs with NAS-181 appeared to slightly decrease the magnitude of the initial suppression (Fig. 6D), but this was not significant (68 ± 4% of control suppression; n = 4 cells; one-factor ANOVA, NS) (Fig. 6G). Coapplication of NAS-181 and ritanserin significantly reduced the magnitude of the suppression (26 ± 9% of control suppression; n = 4 cells; one-factor ANOVA, p < 0.01) (Fig. 6E,G). This suggests that the suppression of EPSCs after stimulation of endogenous serotonin release results from activation of both 5-HT2Rs and 5-HT1BRs (Fig. 6C–E), as was the case for bath application of 5-HTR agonists (Fig. 2). Furthermore, activation of either 5-HT2Rs or 5-HT1BRs is sufficient to cause suppression of this synapse in a manner similar to that for bath application of 5-HTR agonists. Likewise, in the presence of NAS-181 to block 5-HT1BRs, application of AM251 to block CB1Rs significantly reduced the magnitude of the suppression (19 ± 15% of control suppression; n = 4 cells; one-factor ANOVA, p < 0.01) (Fig. 6F,G). This suggests that endogenously released serotonin suppresses EPSCs onto IO cells by activating both 5-HT2Rs and 5-HT1BRs. Moreover, the suppression resulting from 5-HT2R activation is mediated by CB1Rs.
The extent of suppression after activation of serotonergic inputs is less than the maximal suppression observed during bath application of either 10 μm serotonin or the artificial agonist TCB-2. Bath application of 500 nm of exogenous serotonin resulted in a suppression of this synapse to 58 ± 4% of control (n = 4 cells), which is slightly less suppression than that observed after stimulation of serotonin release in the slice (40 ± 2% of control; n = 16 cells). This suggests that the concentration of serotonin accumulating in the slice after stimulation of serotonergic brainstem nuclei is likely slightly >500 nm, although factors such as receptor desensitization could complicate a comparison of the effects of prolonged bath application with transient serotonin signals generated by synaptic activation.
Discussion
We found that serotonin can activate postsynaptic 5-HT2Rs and evoke endocannabinoid release that acts retrogradely on the presynaptic terminal to reduce the probability of glutamate release. This finding links the serotonin and cannabinoid signaling systems and may be an important mechanism of serotonin modulation in the inferior olive and in other brain regions.
Serotonin in the IO
We have shown that serotonin can almost eliminate excitatory synaptic transmission onto dPO neurons. 5-HT2Rs and 5-HT1BRs are both involved in reducing the probability of release from the presynaptic terminals. In response to bath application of a serotonin receptor agonist, antagonists of either 5-HT2Rs or 5-HT1BRs alone only partially relieve the suppression. These findings indicate that activation of either type of receptor is sufficient to reduce synaptic transmission to <45% of control. The important role of both receptors was also apparent in experiments in which modulation was produced by activating serotonergic inputs to the dPO. One of the interesting aspects of the modulation observed under these conditions is that the blockade of either receptor alone has so little effect on the extent of modulation. Blockade of 5-HT1BRs may have slightly decreased the extent of inhibition immediately after the conditioning train, but the effect was small and suppression at later times was unaffected. One complicating factor is the apparent role of 5-HT autoreceptors in control of transmitter release from some serotonergic terminals (Sari, 2004; Sharp et al., 2007). As a result, a 5-HTR antagonist could potentially increase the release of serotonin in addition to influencing excitatory synapses directly. Regardless of the potential for regulation of serotonin release, it is clear that 5-HT2Rs and 5-HT1BRs can both independently regulate excitatory synapses when serotonin is liberated by synaptic stimulation.
This is the first demonstration that serotonin can regulate synapses in a retrograde manner by activating 5-HT2Rs to evoke endocannabinoid release. The modulation by 5-HT2Rs was studied in isolation by blocking 5-HT1BRs.
5-HT2R effects in the IO likely primarily result from activation of 5-HT2ARs because these receptors are expressed at high levels on the soma and dendrites of IO cells, whereas the other two 5-HT2R subtypes, 5-HT2B and 5-HT2C, are not thought to be present (Fay and Kubin, 2000; Fonseca et al., 2001; Leysen, 2004). Despite the postsynaptic location of 5-HT2ARs, the suppression arising from activation of these receptors is expressed presynaptically. This suggests that a retrograde messenger is involved. For both exogenous serotonin receptor agonists and synaptically evoked serotonin release, suppression resulting from activation of 5-HT2Rs requires endocannabinoid activation of CB1Rs. This suggests that the retrograde messenger is an endocannabinoid, and depolarization-induced suppression of excitation experiments establish that IO cells can release endocannabinoids.
Synaptic modulation in the IO
The observation that serotonin suppresses excitatory synapses onto IO cells may help to explain previous results from in vivo experiments related to motor learning and motor activity. Previously, it was shown that injection of serotonin into the IO increased the synchrony of complex spikes recorded from Purkinje cells (Sugihara et al., 1995). As serotonergic brainstem neurons increase their firing during repetitive movements (Jacobs and Fornal, 1997), serotonin may promote synchronous activation of IO cells during repetitive motor activity by depolarizing IO cells and suppressing excitatory synaptic input. In addition, serotonin has been implicated in modulation of motor activity. For instance, systemically altering serotonin levels alters motor output (Jacobs and Fornal, 1997). Our findings suggest that suppression of excitatory synapses within the IO is likely to contribute to the regulation of motor activity by serotonin.
Our findings also provide the first evidence that IO cells can release endocannabinoids and suppress their excitatory synaptic inputs by activating presynaptic CB1Rs. CB1R knock-out mice have deficits in delay eye-blink conditioning, which is a form of learning known to rely on cerebellar circuits (Kishimoto and Kano, 2006). One possible explanation is that endocannabinoids are required for long-term depression in the cerebellar cortex (Safo et al., 2006). However, climbing fibers convey the activity from the IO to the cerebellar cortex and play a crucial role in cerebellar learning (Raymond et al., 1996; Thompson, 2005). Therefore, it is possible that alterations in CB1R signaling in the IO could contribute to deficits in delay eye-blink conditioning.
Implications for 5-HT2R signaling in other brain regions
The demonstration that, within the IO, activation of 5-HT2Rs acts in part by promoting the release of endocannabinoids has important implications for understanding how 5-HT2Rs could function in other brain regions. Studies in humans and animal models suggest that alterations in central serotonin levels affect depression and anxiety-related behaviors. One of the more likely targets for this modulation is 5-HT2Rs (Van Oekelen et al., 2003; Weisstaub et al., 2006). 5-HT2Rs are also thought to play an important role in schizophrenia, epilepsy, and sleep (Van Oekelen et al., 2003; Leysen, 2004; Weisstaub et al., 2006). 5-HT2ARs and the closely related 5-HT2CRs are highly expressed throughout the brain (Leysen, 2004). Activation of cortical 5-HT2Rs is thought to mediate many of the hallucinogenic and mood-altering effects of drugs such as LSD (lysergic acid diethylamide) (Aghajanian and Marek, 1999; Gonzalez-Maeso et al., 2007). Cortical 5-HT2Rs are also thought to play an important role in some psychiatric disorders because a number of antidepressant and antipsychotic drugs target these receptors (Gray and Roth, 2001; Leysen, 2004). For instance, blockade of 5-HT2Rs is thought to enhance the therapeutic actions of selective serotonin reuptake inhibitors on depression (Marek et al., 2003; Celada et al., 2004).
Likewise, CB1Rs are expressed widely and at a very high density throughout the CNS (Tsou et al., 1998; Freund et al., 2003; Chevaleyre et al., 2006). Activation of cannabinoid receptors with exogenous agonists including THC (tetrahydrocannabinol), the active ingredient in marijuana, affects both mood and perception (Chevaleyre et al., 2006). Furthermore, many areas contain both 5-HT2Rs and CB1Rs, including the neocortex, portions of the olfactory system, hippocampus, amygdala, and brainstem nuclei (Tsou et al., 1998; Barnes and Sharp, 1999; Hoyer et al., 2002; Freund et al., 2003; Leysen, 2004; Chevaleyre et al., 2006). Our findings suggest that serotonin could act in part by promoting the release of endocannabinoids, thereby leading to suppression of synapses. More generally, our findings suggest a link between signaling by 5-HT2Rs and CB1Rs.
Footnotes
This work was supported by National Institutes of Health Grant R01DA024090 (W.G.R.). We thank Claudio Acuna-Goycolea, Miklos Antal, Megan Carey, John Crowley, Diasynou Fioravante, Andreas Liu, Michael Myoga, and Todd Pressler for comments on this manuscript.
References
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Woodward DJ. Inferior olivary nuclear complex of the rat: morphology and comments on the principles of organization within the olivocerebellar system. J Comp Neurol. 1987;263:467–484. doi: 10.1002/cne.902630402. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH. Cell bodies of origin of serotonin-immunoreactive afferents to the inferior olivary complex of the rat. Brain Res. 1986;399:369–373. doi: 10.1016/0006-8993(86)91530-1. [DOI] [PubMed] [Google Scholar]
- Bourrat F, Sotelo C. Postnatal development of the inferior olivary complex in the rat. I. An electron microscopic study of the medial accessory olive. Brain Res. 1983;284:291–310. doi: 10.1016/0165-3806(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Caridha D, Yourick D, Cabezas M, Wolf L, Hudson TH, Dow GS. Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin. Antimicrob Agents Chemother. 2008;52:684–693. doi: 10.1128/AAC.00874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Coker SJ, Batey AJ, Lightbown ID, Diaz ME, Eisner DA. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. Br J Pharmacol. 2000;129:323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclin JC, Colin F. The olivocerebellar system. II. Some ultrastructural correlates of inferior olive destruction in the rat. Brain Res. 1980;187:29–46. doi: 10.1016/0006-8993(80)90492-8. [DOI] [PubMed] [Google Scholar]
- de Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Mesodiencephalic and cerebellar terminals terminate upon the same dendritic spines in the glomeruli of the cat and rat inferior olive: an ultrastructural study using a combination of [3H]leucine and wheat germ agglutinin coupled horseradish peroxidase anterograde tracing. Neuroscience. 1990;34:645–655. doi: 10.1016/0306-4522(90)90171-y. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- Fonseca MI, Ni YG, Dunning DD, Miledi R. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Brain Res Mol Brain Res. 2001;89:11–19. doi: 10.1016/s0169-328x(01)00049-3. [DOI] [PubMed] [Google Scholar]
- Foster RE, Peterson BE. The inferior olivary complex of guinea pig: cytoarchitecture and cellular morphology. Brain Res Bull. 1986;17:785–800. doi: 10.1016/0361-9230(86)90090-0. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Adams JC, Mugnaini E. GABAergic neurons in the mammalian inferior olive and ventral medulla detected by glutamate decarboxylase immunocytochemistry. J Comp Neurol. 1992;321:501–514. doi: 10.1002/cne.903210402. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Davis TM, Higham CE, Clark A, Ashcroft FM. The antimalarial agent mefloquine inhibits ATP-sensitive K-channels. Br J Pharmacol. 2000;131:756–760. doi: 10.1038/sj.bjp.0703638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen XL, Wang L, Rampe D. Interactions of the antimalarial drug mefloquine with the human cardiac potassium channels KvLQT1/minK and HERG. J Pharmacol Exp Ther. 2001;299:290–296. [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26:8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol (Lond) 1981a;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol (Lond) 1981b;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28:402–412. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem. 2006;99:1164–1175. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- Placantonakis DG, Schwarz C, Welsh JP. Serotonin suppresses subthreshold and suprathreshold oscillatory activity of rat inferior olivary neurones in vitro. J Physiol (Lond) 2000;524:833–851. doi: 10.1111/j.1469-7793.2000.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Safo PK, Cravatt BF, Regehr WG. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5:134–145. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Liu X, Chen C. Frequency-dependent modulation of retinogeniculate transmission by serotonin. J Neurosci. 2004;24:10950–10962. doi: 10.1523/JNEUROSCI.3749-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the “post”: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Lang EJ, Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats: a study using the retrograde transport of horseradish peroxidase. Am J Anat. 1983a;166:329–341. doi: 10.1002/aja.1001660307. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats. An anterograde study using autoradiographic and axonal degeneration techniques. Neuroscience. 1983b;8:259–275. doi: 10.1016/0306-4522(83)90064-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Sano Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the inferior olivary complex of the rat, cat and monkey. Cell Tissue Res. 1983;231:17–28. doi: 10.1007/BF00215770. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21(RC188):1–5. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]