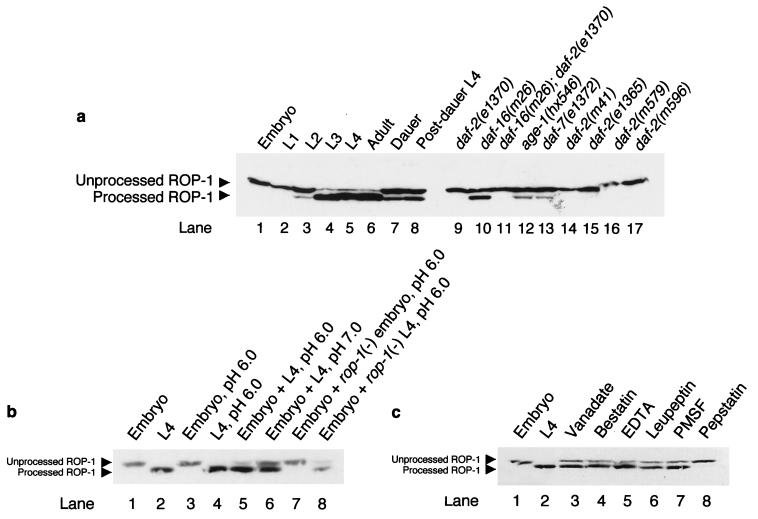
Figure 2.
Dauer genes regulate the processing of ROP-1 by an aspartic proteinase during C. elegans larval development. (a) Western blot analysis of ROP-1 on 100 μg of total protein extract from staged animals revealed that ROP-1 undergoes a mobility change during C. elegans larval development. In the wild type, the mobility shift of ROP-1 occurs at the L2–L3 stage transition (lanes 3 and 4). This mobility shift is influenced by components of the dauer formation pathway (lanes 9–17). All mutant extracts were obtained from L4 animals grown at 20°C in the same conditions as wild-type animals and, thus, should be compared with the wild-type L4 extract (lane 5). (b) The in vitro reconstitution of the ROP-1 processing activity revealed that although embryo extracts alone did not contain the activity (lane 3 and 7), addition of L4 extracts from wild type or rop-1(−) was sufficient to allow the processing of ROP-1 at pH 6.00 (lanes 5 and 8). Increasing the pH to 7.00, completely inhibited the processing of ROP-1 (lane 6). (c) The in vitro reconstitution of the ROP-1 processing activity in the presence of several inhibitors for various modification enzymes demonstrated that inhibitors of phosphatases, exoproteases, serine proteases, cysteine proteases, and metalloproteases had no effect on the processing of ROP-1 (lanes 3–7, respectively). However, addition of a specific inhibitor of aspartic proteinases (Pepstatin, lane 8) efficiently inhibited the processing reaction, demonstrating that ROP-1 is processed by an aspartic proteinase. Processed and unprocessed forms of ROP-1 are indicated.