Fig. 3.
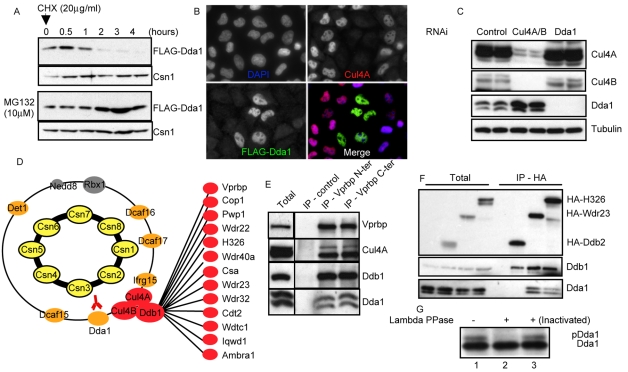
Dda1 is an unstable core subunit of multiple CRL4s. (A) HEK 293T cells stably expressing FLAG-Dda1 were treated with MG132 or for control DMSO. After 3 hours, protein translation was inhibited with cycloheximide (CHX) and samples were collected at various times (in hours) after CHX addition. Protein extracts were analyzed by SDS-PAGE with specific FLAG and Csn1 antibodies. (B) HeLa cells incubated with oligonucleotides targeting Cul4A were transfected with a plasmid expressing FLAG-Dda1. Cells were fixed and immunostained with anti-Cul4A (upper right panel) and anti-FLAG antibodies (bottom left panel). DAPI was used to counterstain DNA (upper left panel), and merged images are presented in the bottom right panel. (C) Protein extracts prepared from cells incubated with control oligonucleotides (lanes 1 and 2), or oligonucleotides targeting Dda1 (lanes 3 and 4) or Cul4 paralogs (lanes 5 and 6) were separated by SDS-PAGE. Cul4A and B, Cdt1, and Dda1 levels were assessed by immunoblotting using specific antibodies. Tubulin was used as a loading control. (D) LC-MS-MS analysis of anti-FLAG-Dda1 immunoprecipitates. Note that FLAG-Dda1 co-purifies with all COP9 signalosome (subunits highlighted in yellow) and many CRL4 subunits (colored in red and orange). (E) Vprbp was immunoprecipitated with control antibodies or antibodies directed against the N- or C-terminal part of the protein. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with specific antibodies specific for Vprbp, Cul4A, Ddb1 and Dda1. (F) HA-tagged DCAF proteins H326, Wdr23 and Ddb2 were expressed in HeLa cells and immunoprecipitated. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with specific antibodies directed against the HA peptide, Ddb1 and Dda1. (G) Dda1 immunoprecipitates were treated with lambda phosphatase (lane 2) or an inactivated phosphatase (lane 3) and analyzed by western blotting using specific Dda1 antibodies.