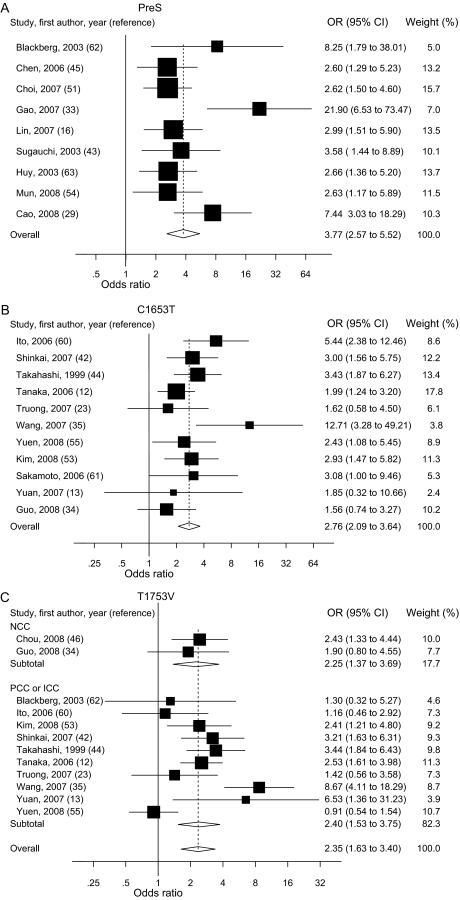
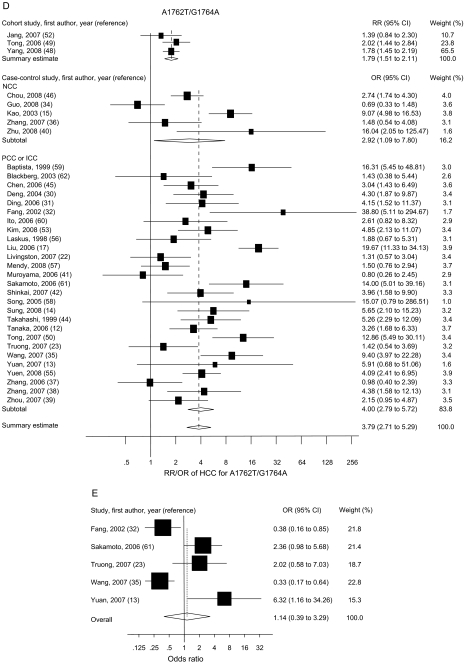
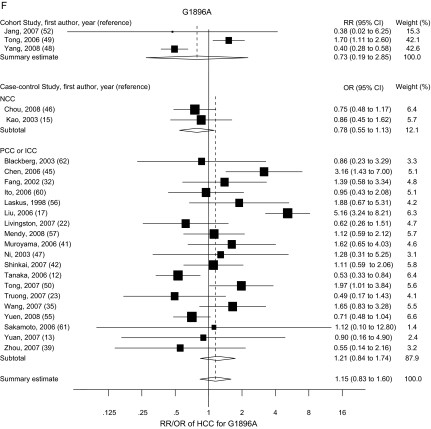
Figure 2.
Summary odds ratios (ORs; in case–control studies) or relative risks (RRs; in cohort studies) of hepatocellular carcinoma (HCC) for PreS (A), C1653T (B), T1753V (C), A1762T/G1764A (D), C1858T (E), and G1896A (F) mutations. Squares represent study-specific estimates (size of the square reflects the study-specific statistical weight); horizontal lines represent 95% confidence intervals (CIs); diamonds represent summary estimates with corresponding 95% CIs. Test for heterogeneity: (A) P = .045, I2 = 49.3%; (B) P = .197, I2 = 25.9%; (C) P < .001, I2 = 66.3%; (D) P < .001, I2 = 76.4%; (E) P < .001, I2 = 83.2%; (F) P < .001, I2 = 80.0%. A random-effects model was used for all analyses. All statistical tests were two-sided. NCC = nested case–control; PCC = prevalence case–control; ICC = incidence case–control.