Abstract
AIM: To explore the effect of Astragalus mongholicus polysaccharide (APS) on gene expression and mitogen-activated protein kinase (MAPK) transcriptional activity in intestinal epithelial cells (IEC).
METHODS: IEC were divided into control group, lipopolysaccharide (LPS) group, LPS+ 50 μg/mL APS group, LPS+ 100 μg/mL APS group, LPS+ 200 μg/mL APS group, and LPS+ 500 μg/mL APS group. Levels of mRNAs in LPS-induced inflammatory factors, tumor necrosis factor (TNF)-α and interleukin (IL)-8, were measured by reverse transcription-polymerase chain reaction. MAPK protein level was measured by Western blotting.
RESULTS: The levels of TNF-α and IL-8 mRNAs were significantly higher in IEC with LPS-induced damage than in control cells. APS significantly abrogated the LPS-induced expression of the TNF-α and IL-8 genes. APS did not block the activation of extracellular signal-regulated kinase or c Jun amino-terminal kinase, but inhibited the activation of p38, suggesting that APS inhibits LPS-induced production of TNF-α and IL-8 mRNAs, possibly by suppressing the p38 signaling pathway.
CONCLUSION: APS-modulated bacterial product-mediated p38 signaling represents an attractive strategy for prevention and treatment of intestinal inflammation.
Keywords: Astragalus mongholicus polysaccharide, Intestinal epithelial cells, Tumor necrosis factor-α, Interleukin-8, Extracellular signal-regulated kinase, C Jun amino-terminal kinase, p38 kinase
INTRODUCTION
Intestinal epithelia cells (IEC) are the first line of defense against noxious intraluminal agents, including microorganisms and toxic antigens[1]. Although IEC are less responsive to polysaccharide than monocytes/macrophages, it has been shown that endotoxin triggers a proinflammatory gene transcriptional program in some IEC[2], including the rat small intestinal cell line IEC-6[1,3,4]. Luminal endotoxin may participate in various intestinal inflammatory disorders. Modulation of bacteria- and bacterial product-induced gene expression in the intestine may have a significant impact on intestinal inflammatory disorders[5].
Astragali Radix, root of Astragalus membranaceus Bunge, is a popular herb that has been used for thousands of years in treatment of a variety of diseases in oriental medicine. Astragalus mongholicus polysaccharide (APS) is the main ingredient of Astragali Radix. Studies have revealed the anti-inflammatory, antioxidant, and immune regulatory roles of APS[6]. However, knowledge of how APS exerts its anti-inflammatory effects is still limited. Lee et al[7] reported that Astragali Radix appears to exert immune modulating effects by regulating the expression of cytokines, such as interleukin (IL)-1, IL-6 and inducible nitric oxide synthase (iNOS), as well as the production of nitric oxide (NO). In this study, the effect of APS on LPS-induced mitogen-activated protein kinase (MAPK) signaling and pro-inflammatory gene expression in IEC-6 cells was investigated, showing that APS prevents the activation of p38MAPK signaling in IEC-6 cells in vitro.
MATERIALS AND METHODS
Materials
APS was isolated from a 6-year-old Astragalus membranaceus sample purchased from the Chinese Medicinal Herbs Company (Beijing, China), with a purity of 98.5%. IEC-6 cells were purchased from the Chinese Academy of Medical Sciences, Center for Biological Detection (Beijing, China). Lipopolysaccharide (LPS, Escherichia coli O55:B5) and insulin (I5500) were purchased from Sigma (USA). Phospho-specific rabbit polyclonal antibodies against Thr180 and Tyr182 dual-phosphorylated p38, Thr183 and Tyr185 dual-phosphorylated c Jun amino-terminal kinase (JNK), Thr202 and Tyr204 dual-phosphorylated extracellular signal-regulated kinase (ERK)/2 and total p38, ERK1/2, JNK were purchased from Cell Signaling Technology (USA). A rabbit polyclonal antibody against actin and a peroxidase (HRP)-labeled anti-rabbit IgG antibody were purchased from Sigma (USA).
Culture and treatment of IEC
The rat small intestinal cell line IEC-6 was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 0.01 mg/mL insulin. IEC-6 cells were grown in 6-well plates at a density of 5 × 105 cells per well and cultured in DMEM at 37°C in a humidified atmosphere containing 50 mL CO2 for 24 h. After incubation, non adherent cells were removed and adherent cells were pretreated for 1 h with APS at different concentrations (50, 100, 200 and 500 μg/mL). The cells were then stimulated with LPS (10 μg/mL) and harvested at the indicated time points.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
IEC-6 cells were cultured in DMEM containing LPS with or without various concentrations of APS, for 1 h to allow detection of tumor necrosis factor (TNF)-α mRNA, and for 2 h to allow detection of IL-8 mRNA. Cells were washed in PBS and used for RNA isolation. Total RNA was isolated using Trizol reagent according to its manufacturer’s instructions. RT-PCR was carried out using 1 μg of total RNA from IEC-6 cells and an oligo(dT)12-18 primer.
The sequences of primers for amplification of cDNAs of rat TNF-α-U, TNF-α-L, IL-8-L, GAPDH-U and GAPDH-L are 5'-TTCGGGGTGATCGGTCCCAA-3', 5'-AGCATCTCGTGTGTTTCTGA-3', 5'-CCTGAAGACCCTACCAAG-3', AGGCTCCATAAATGAAAGA-3', 5'-ATCACTGCCACTCAGAAGAC-3', 5'-TGAGGGAGATGCTCAGTGTT-3', respectively. GAPDH was used as an invariant housekeeping internal control gene. Twenty-five cycles of amplification were performed for all reactions. The length of PCR products of TNF-α, IL-8 and GAPDH was 750, 494 and 580 bp, respectively.
Western blotting analysis
IEC-6 cells were stimulated with LPS (10 μg/mL) for various periods of time (0-1 h). The cells were cultured in a medium containing LPS with or without various concentrations of APS for 1 h to detect phosphorylated-p38, ERK1/2, JNK, and total p38, ERK, and JNK, and lysed with a SDS sample buffer. The supernatants were analyzed by 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, which were blocked with 10% nonfat dry milk in TBST containing 20 mmol/L Tris (pH 8.0), 137 mmol/L NaCl and 10% Tween-20, and blotted with the relevant primary antibody, then with a horseradish peroxidase-conjugated secondary antibody. Bound proteins were detected by enhanced chemiluminescence according to its manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SPSS 11.5. All data were expressed as mean ± SE. Statistical significance of differences among values was determined by ANOVA and LSD was used for inter-group comparison. P < 0.05 was considered statistically significant.
RESULTS
APS abrogated LPS-induced TNF-α and IL-8 gene expression in IEC-6 cells
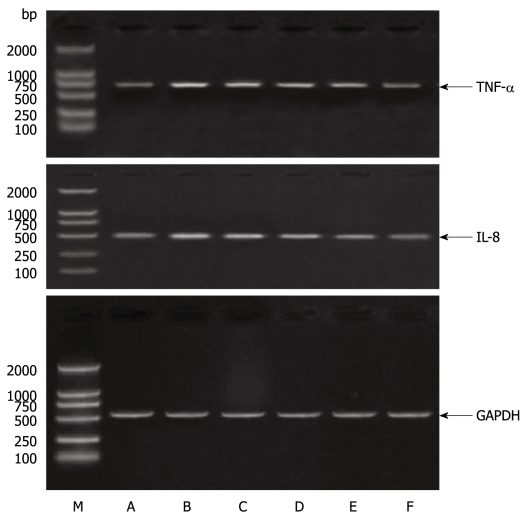
The effects of APS on LPS-induced TNF-α and IL-8 gene expression in the intestinal cell line IEC-6 were evaluated. Stimulation of IEC-6 cells by LPS markedly increased the production of TNF-α and IL-8. The effect of APS on the levels of TNF-α and IL-8 mRNAs in IEC-6 cells was detected after LPS stimulation. IEC-6 cells were pretreated with APS at different concentrations (50, 100, 200 and 500 μg/mL) for 24 h, stimulated with LPS (10 μg/mL) for 1 h. TNF-α and IL-8 gene expressions were detected by RT-PCR. As shown in Figure 1, RT-PCR analysis revealed that TNF-α and IL-8 mRNAs were induced readily in IEC-6 cells by LPS. However, this induction was inhibited by APS in a concentration-dependent manner, namely 50 and 500 μg/mL APS partially and significantly suppressed the production of TNF-α and IL-8 mRNAs in the presence of LPS-activated IEC-6 cells compared with LPS stimulation in the absence of APS (P < 0.01).
Figure 1.
Astragalus mongholicus polysaccharide inhibits TNF-α and IL-8 production in LPS-stimulated rat small intestinal cells. Intestinal epithelial cells (IEC) were treated with APS for 1 h, and cultured in a medium containing 10 μg/mL. LPS with APS at different concentrations for 1 h to detect TNF-α and IL-8 mRNAs in the cells by RT-PCR. M: Marker. A: Control group; B: LPS+ 0 μg/mL APS group; C: LPS+ 50 μg/mL APS group; D: LPS+ 100 μg/mL APS group; E: LPS+ 200 μg/mL APS group; F: LPS+ 500 μg/mL APS group.
APS inhibited both TNF-α and IL-8 production by LPS-activated IEC-6 cells in a time-dependent manner
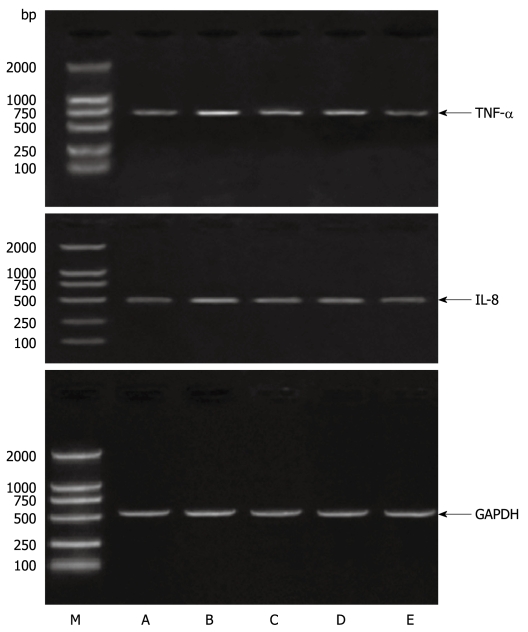
IEC-6 cells were pretreated with APS (500 μg/mL) for 24 h, stimulated with LPS (10 μg/mL) for 1-4 h. The expression of the TNF-α and IL-8 genes was detected by RT-PCR. As shown in Figure 2, LPS-induced TNF-α mRNA expression was inhibited 10.3% and 25.5% by APS treatment at 1 and 4 h post-stimulation, respectively. LPS-induced IL-8 mRNA expression was also inhibited 15.3% and 18.8% by APS treatment at 1 and 4 h post-stimulation, respectively.
Figure 2.
Astragalus mongholicus polysaccharide inhibits TNF-α and IL-8 production in LPS-stimulated rat small intestinal cells. IEC were treated with APS (500 μg/mL) for 1 h, and cultured in a medium containing 10 μg/mL. LPS for up to 4 h to detect TNF-α and IL-8 mRNAs in the cells by RT-PCR. M: Marker. A: Control group; B: LPS+ 0 μg/mL APS group; C: LPS+ 50 μg/mL APS group; D: LPS+ 100 μg/mL APS group; E: LPS+ 200 μg/mL APS group; F: LPS+ 500 μg/mL APS group.
APS inhibited p38 phosphorylation but not ERK1/2 or JNK activation in LPS–activated IEC-6 cells
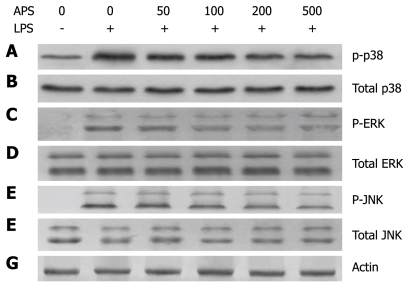
Activation of MAPK p38 can mediate the production of pro-inflammatory cytokines when IEC-6 cells are activated. To further understand the mechanisms underlying the APS-mediated anti-inflammation in IEC-6 cells, we examined whether APS inhibits LPS-triggered activation of MAPK signaling, including phosphorylation of p38, ERK1/2 and JNK. The levels of phosphorylated (activated) p38, ERK1/2 and JNK were analyzed in LPS-stimulated IEC-6 cells following treatment with or without APS. p38, ERK1/2 and JNK were strongly activated in IEC-6 cells stimulated with LPS. However, APS treatment decreased LPS-induced p38 phosphorylation. Moreover, p38 phosphorylation was inhibited by LPS in a concentration-dependent manner. Fifty micrograms per millilitre APS partially blocked LPS-induced p38 phosphorylation and 500 μg/mL APS significantly inhibited p38 phosphorylation in LPS-stimulated IEC-6 cells (Figure 3) while the total protein level of p38, ERK1/2 or JNK remained unchanged, indicating that APS inhibits the activation of p38 but not ERK1/2 and JNK in IEC-6 cells after LPS stimulation.
Figure 3.
Astragalus mongholicus polysaccharide inhibits p38 phosphorylation but not ERK1/2 or JNK activation in LPS-stimulated rat small intestinal cells. IEC were treated as described in Figure 1. After incubation in a medium containing 10 μg/mL LPS with APS at different concentrations of for 1 h, Western blotting analysis was performed to detect phosphorylated p38 (A), total p38 (B), phosphorylated ERK (C), total ERK (D), phosphorylated JNK (E), total JNK (F) and actin (G).
DISCUSSION
The search for active compounds in natural products used in traditional medicine has attracted great interest, because traditional herbal drugs have many benefits, few side effects and low cytotoxicity[8–11]. Isolation, identification and characterization of these compounds, and evaluation of their potential benefits to humans, have become an important field in pharmaceutical research[12].
It has been reported that APS has a variety of pharmacological properties. Traditionally, APS is used to treat weakness, wound, anemia, fever, multiple allergies, chronic fatigue, and loss of appetite[13]. APS is used as a diuretic and tonic herbal medicine in Asian countries, to enhance physical strength and endurance, strengthen the immune system, decrease blood pressure, and promote excretion and circulation[6,14,15]. Clinically, APS is used to treat chronic phlegmatic disorders and general gastrointestinal disturbances including stomach ulcer and diarrhea[16,17]. The mechanism by which APS mediates the above-mentioned effects is unclear. Studies on the use of APS in treatment of various human diseases showed that this herb may act as an immune regulator that can enhance strength, immunity and circulation[18–21]. It has been reported that APS also appears to exert an immune modulating effect by regulating the expression of cytokines such as IL-1, IL-6 and iNOS, as well as the production of NO[6]. In this study, APS inhibited the production of both TNF-α and IL-8 in LPS-stimulated IEC-6 cells in a concentration-dependent manner (Figure 1). Since excessive production of TNF-α and IL-8 induces tissue injury, septic shock and inflammatory intestinal disease, APS can be developed into a drug for intestinal injury.
MAPKs (ERK, p38, JNK)[11,22–24] and NF-κB[25–27] positively control TNF-α and IL-8 expression in LPS-activated IEC-6 cells, via a unique signaling pathway. Inhibition of any of the three MAPK pathways is sufficient to block the TNF-α and IL-8 induced by LPS in IEC-6 cells[28–30]. In this study, whether APS exerts its effects on TNF-α and IL-8 by interfering with the activation of ERK, p38 and JNK was tested, showing that APS cannot block the activation of ERK or JNK. Therefore, these two pathways do not mediate any inhibitory effect of APS on TNF-α and IL-8 production by LPS-stimulated IEC-6 cells. In our study, APS inhibited the activation of p38 and the expression of the TNF-α and IL-8 genes, suggesting that inhibition of the activation of p38 but not ERK and JNK may inhibit the production of TNF-α and IL-8.
In summary, APS inhibits the production of both TNF-α and IL-8 in LPS-stimulated IEC-6 cells by suppressing p38 signaling.
COMMENTS
Background
Astragalus mongholicus polysaccharide (APS) with a variety of pharmacological properties is a component isolated from Astragali Radix, a traditional Chinese herbal medicine. Studies have revealed its anti-inflammatory, antioxidant, and immune regulatory effects. However, knowledge about how APS exerts its anti-inflammatory effects is still limited.
Research frontiers
Astragali Radix appears to exert its immune modulating effects by regulating the expression of cytokines, such as interleukin (IL)-1, IL-6 and inducible nitric oxide synthase, as well as the production of nitric oxide. Thus, whether APS affects the production of tumor necrosis factor (TNF)-α and IL-8 in lipopolysaccharide (LPS)-activated intestinal epithelial cells (IEC)-6 cells by interfering with mitogen-activated protein kinase (MAPK) signaling was investigated in this study.
Innovations and breakthroughs
This is the first study to investigate the effect of APS on LPS-induced MAPK signaling and pro-inflammatory gene expression in IEC-6 cells. APS was found to inhibit the production of both TNF-α and IL-8 in LPS-induced IEC-6 cells in a concentration-dependent manner, and excessive production of TNF-α and IL-8 was observed to induce tissue injury, septic shock and inflammatory intestinal disease.
Applications
APS can be developed into a drug for intestinal injury.
Terminology
MAPK phosphorylates serine and threonine residues of proteins in cells. MAPK is also an important signal regulator linking cell surface receptors to changes in gene expression. In mammalian cells, at least three members of the MAPK family including extracellular signal-regulated kinase (ERK), c Jun amino-terminal kinase (JNK), and p38 have been cloned.
Peer review
In this study, the authors detected the protective effects of a purified herbal product on LPS-induced intestinal inflammatory mucosal injury and conducted their study in IEC-6 cells and measured the levels of TNF-α and IL-8 mRNAs as well as proteins in the p38 signaling pathway. The results presented in this paper show that purified herbal polysaccharide can inhibit LPS-induced production of TNF-α and IL-8 by suppressing p38, P-ERK and P-JNK. In general, the experiments in this study were carefully designed and carried out. Data description is clear and the results are adequately discussed.
Peer reviewers: Jian-Ying Wang, Professor, University of Maryland School of Medicine, Baltimore VA Medical Center (112), 10N. Greene St, Baltimore, MD 21201, United States; Alain L Servin, PhD, Faculty of Pharmacy, French National Institute of Health and Medical Research, Unit 756, Rue J.-B. Clément, F-922296 Châtenay-Malabry, France
S- Editor Tian L L- Editor Wang XL E- Editor Zheng XM
References
- 1.Haller D, Jobin C. Interaction between resident luminal bacteria and the host: can a healthy relationship turn sour? J Pediatr Gastroenterol Nutr. 2004;38:123–136. doi: 10.1097/00005176-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lotz M, Ménard S, Hornef M. Innate immune recognition on the intestinal mucosa. Int J Med Microbiol. 2007;297:379–392. doi: 10.1016/j.ijmm.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Haller D, Holt L, Kim SC, Schwabe RF, Sartor RB, Jobin C. Transforming growth factor-beta 1 inhibits non-pathogenic Gram negative bacteria-induced NF-kappa B recruitment to the interleukin-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J Biol Chem. 2003;278:23851–23860. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
- 4.Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–38178. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol. 2005;141:288–297. doi: 10.1111/j.1365-2249.2005.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao KS, Mancini C, Doria G. Enhancement of the immune response in mice by Astragalus membranaceus extracts. Immunopharmacology. 1990;20:225–233. doi: 10.1016/0162-3109(90)90038-g. [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Han OK, Park CW, Yang CH, Jeon TW, Yoo WK, Kim SH, Kim HJ. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells. J Ethnopharmacol. 2005;100:289–294. doi: 10.1016/j.jep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZQ, Li QZ, Qin GJ. [Effect of Astragalus injection on platelet function and plasma endothelin in patients with early stage diabetic nephropathy] Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:274–276. [PubMed] [Google Scholar]
- 9.Wu L, Liu H, Xue P, Lu ZG, Du KF. [Influence of a triplex superimposed treatment on HBV replication and mutation during treating chronic hepatitis B] Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2001;15:236–238. [PubMed] [Google Scholar]
- 10.Yesilada E, Bedir E, Caliş I, Takaishi Y, Ohmoto Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J Ethnopharmacol. 2005;96:71–77. doi: 10.1016/j.jep.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Chen XJ, Bian ZP, Lu S, Xu JD, Gu CR, Yang D, Zhang JN. Cardiac protective effect of Astragalus on viral myocarditis mice: comparison with Perindopril. Am J Chin Med. 2006;34:493–502. doi: 10.1142/S0192415X06004028. [DOI] [PubMed] [Google Scholar]
- 12.Ryu M, Kim EH, Chun M, Kang S, Shim B, Yu YB, Jeong G, Lee JS. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J Ethnopharmacol. 2008;115:184–193. doi: 10.1016/j.jep.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Ha H, Kim JS, Kim YT, Kwon SC, Park SW. Induction of growth hormone by the roots of Astragalus membranaceus in pituitary cell culture. Arch Pharm Res. 2003;26:34–39. doi: 10.1007/BF03179928. [DOI] [PubMed] [Google Scholar]
- 14.Sheng MX, Li JZ, Wang HY. [Therapeutic effect of Astragalus and Angelica on renal injury induced by ischemia/reperfusion in rats] Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:43–46. [PubMed] [Google Scholar]
- 15.Yu J, Zhang Y, Sun S, Shen J, Qiu J, Yin X, Yin H, Jiang S. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Can J Physiol Pharmacol. 2006;84:579–587. doi: 10.1139/y06-015. [DOI] [PubMed] [Google Scholar]
- 16.Yang DZ. [Effect of Astragalus membranaceus on myoelectric activity of small intestine] Zhongguo Zhongxiyi Jiehe Zazhi. 1993;13:616–617, 582. [PubMed] [Google Scholar]
- 17.Hei ZQ, Huang HQ, Zhang JJ, Chen BX, Li XY. Protective effect of Astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J Gastroenterol. 2005;11:4986–4991. doi: 10.3748/wjg.v11.i32.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000;13:523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han SB, Kim YH, Lee CW, Park SM, Lee HY, Ahn KS, Kim IH, Kim HM. Characteristic immunostimulation by angelan isolated from Angelica gigas Nakai. Immunopharmacology. 1998;40:39–48. doi: 10.1016/s0162-3109(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 20.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 21.Cui R, He J, Wang B, Zhang F, Chen G, Yin S, Shen H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51:75–80. doi: 10.1007/s00280-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 23.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 24.Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176:580–588. doi: 10.4049/jimmunol.176.1.580. [DOI] [PubMed] [Google Scholar]
- 25.Haller D, Holt L, Parlesak A, Zanga J, Bäuerlein A, Sartor RB, Jobin C. Differential effect of immune cells on non-pathogenic Gram-negative bacteria-induced nuclear factor-kappaB activation and pro-inflammatory gene expression in intestinal epithelial cells. Immunology. 2004;112:310–320. doi: 10.1111/j.1365-2567.2004.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YS, Kim JS, Jung HC, Song IS. The effects of thalidomide on the stimulation of NF-kappaB activity and TNF-alpha production by lipopolysaccharide in a human colonic epithelial cell line. Mol Cells. 2004;17:210–216. [PubMed] [Google Scholar]
- 27.Sumbayev VV, Yasinska IM. Role of MAP kinase-dependent apoptotic pathway in innate immune responses and viral infection. Scand J Immunol. 2006;63:391–400. doi: 10.1111/j.1365-3083.2006.001764.x. [DOI] [PubMed] [Google Scholar]
- 28.Nimah M, Zhao B, Denenberg AG, Bueno O, Molkentin J, Wong HR, Shanley TP. Contribution of MKP-1 regulation of p38 to endotoxin tolerance. Shock. 2005;23:80–87. doi: 10.1097/01.shk.0000145206.28812.60. [DOI] [PubMed] [Google Scholar]
- 29.Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280:8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]