FIGURE 1.
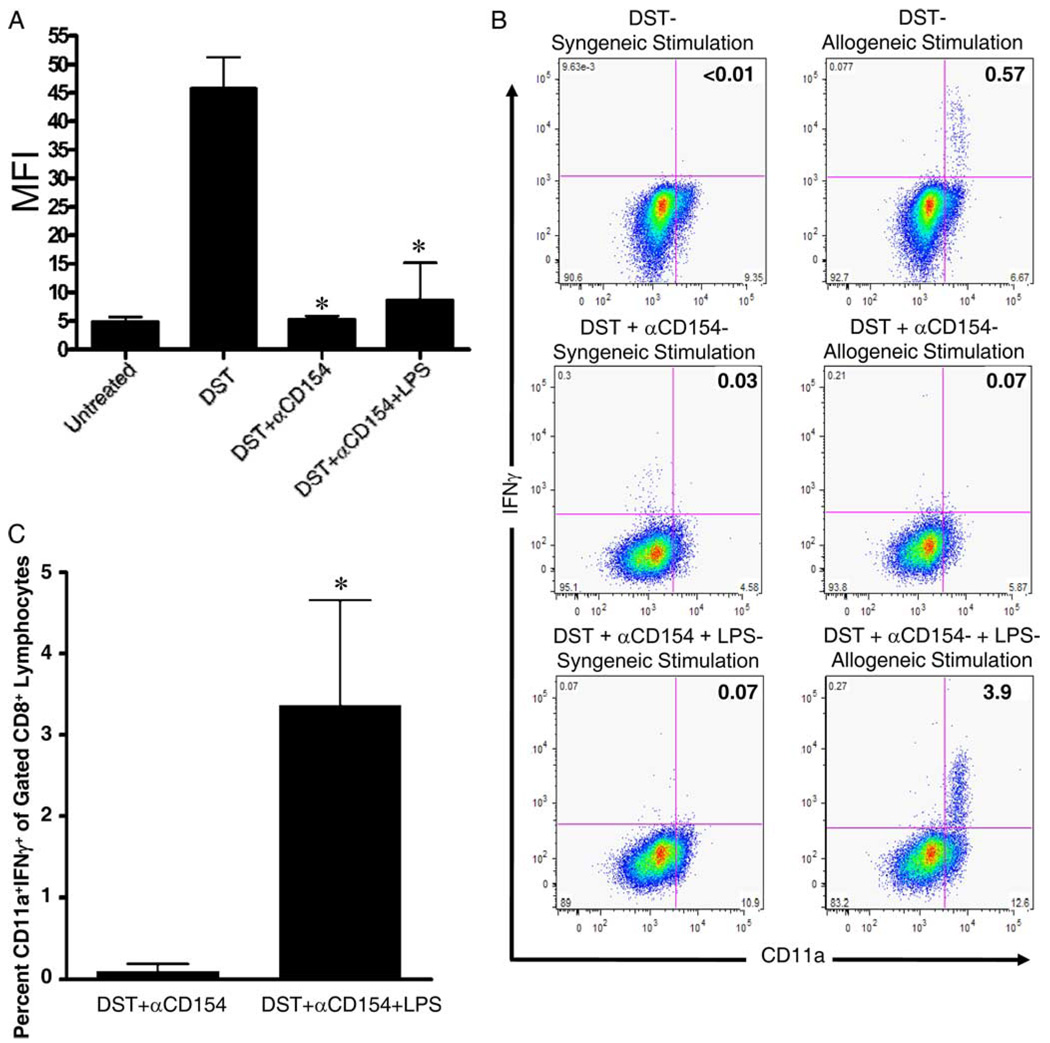
LPS administration at the time of costimulation blockade permits the generation of alloreactive CD8+ T cells but does not induce alloantibody production. A, C57BL/6 mice were treated with BALB/c DST and anti-CD154 mAb according to our standard protocol without or with injection of 100 µg of LPS on day −7 relative to transplantation of BALB/c bone marrow on day 0. All mice were bled 2 wk after transplantation. Serum was analyzed for alloantibody content by flow cytometry. Serum was also taken from untreated mice and mice primed with a single injection of BALB/c splenocytes to serve as negative and positive controls, respectively, for the alloantibody assay. Data are presented as MFI + 1 SD. Data are pooled from two independent experiments with at least four mice per group. *, p < 0.001 vs splenocyte (DST)-injected-only group; p = NS vs untreated group. p = NS for DST + anti-CD154 mAb vs DST + anti-CD154 mAb + LPS. B and C, C57BL/6 mice were treated with BALB/c DST and anti-CD154 mAb according to our standard protocol without or with coinjection of 100 µg of LPS on day −7 relative to transplantation of BALB/c bone marrow and skin on day 0. All mice were bled 2 wk after transplantation. Peripheral blood cells were recovered 2 wk after transplantation and stimulated in vitro for 5 h with either irradiated syngeneic (H2b) or allogeneic (H2d) splenocytes and their production of IFN-γ was quantified by flow cytometry. B, Representative flow cytometry dot plots showing CD11a and IFN-γ expression in gated CD8+ lymphocytes. As a positive control, C57BL/6 mice were injected with BALB/c splenocytes (DST) 7 days before blood cell recovery. C, The mean + 1 SD of the percentage of CD8+ lymphocytes producing IFN-γ. Data are representative of two independent experiments with at least three mice per group. *, p = 0.012.