Abstract
AIM: To determine factors associated with fibrosis progression in hepatitis C virus (HCV)-infected patients without significant initial pathological lesions.
METHODS: Seventy six untreated HCV-infected patients with initially normal liver as defined by a Knodell score ≤ 3, with 2 liver biopsies and detectable HCV-RNA were included. Markers of fibrosis progression were assessed.
RESULTS: Median duration of infection and time between paired biopsies was 13 (95% CI: 1-28) and 4 (95% CI: 2-16) years respectively. Alanine-transaminase (ALT) activity was normal in 43.4% of cases. 50% demonstrated progression of the necro-inflammation and 34% of fibrosis after a median time evolution of 4 years (95% CI: 2-16). The median difference in the necro-inflammation and fibrosis score between biopsies was low, 1.5 and 0.0 respectively. Univariate analysis showed there was no difference between fibrosis activity or evolution according to genotype or viral load. A higher fibrosis progression (P = 0.03) was observed in patients with body mass index (BMI) > 25. Fibrosis progression correlated with the time interval between biopsies (P = 0.01). A significant progression of activity (1.7 vs 0.4, P < 0.05) or fibrosis (0.9 vs 0.0, P < 0.01) was observed in patients with elevated ALT. There was a significant correlation between activity progression and fibrosis progression (P = 0.003). Multivariate analysis demonstrated that fibrosis progression was associated with elevated ALT, BMI > 25 and the time interval between 2 biopsies.
CONCLUSION: There is no fibrosis progression in 66% of patients without significant initial histopathological lesion. Fibrosis progression is associated with elevated ALT and BMI > 25.
Keywords: Hepatitis C virus, Liver fibrosis, Liver biopsy, Alanine-transaminase, Body mass index
INTRODUCTION
Several studies have evaluated the long-term outcome of fibrosis progression in patients with chronic active hepatitis. A normal liver is observed in about 10% of infected patients as defined by the absence of significant pathological changes[1–3]. The natural history of these so-called “healthy hepatitis C virus (HCV) carriers” is not fully defined. Studies usually differentiate patients with or without alanine-transaminase (ALT) abnormalities but rarely patients with or without liver biopsy abnormalities[4]. It is estimated that 10% to 40% of HCV-infected patients have a carrier state with normal ALT and a slower natural course of the disease than patients with elevated ALT[5–9]. The aim of this study was to better define the pathological spontaneous evolution in HCV-infected immunocompetent patients without specific histopathological lesions.
MATERIALS AND METHODS
Among our file of 3600 HCV-infected patients in the liver unit, we selected retrospectively those fulfilling the following criteria: detectable HCV RNA, Knodell score ≤ 3 at the first pathological evaluation[10], at least 2 sequential liver biopsies in the absence of antiviral therapy or HIV and HBV confection. Kidney transplant recipients or hemodialyzed patients were excluded as well as patients with other causes of chronic liver disease (hepatotoxic drugs, autoimmune chronic hepatitis, hemochromatosis, Wilson’s disease and alpha 1 antitrypsin deficiency). In our center we usually perform a liver biopsy in all HCV-RNA chronic carriers, whatever the transaminase levels. In the case of low pathological lesions, we usually propose therapeutic abstention, a biochemical follow-up twice a year, an abdominal ultrasonography (US) yearly and a pathological follow-up with a liver biopsy every three to five years. In patients infected by blood transfusion or intravenous drug use, duration of HCV infection was estimated as the time elapsed from the year of transfusion or intravenous drug use (IVDU) onset to that of the first liver biopsy. Gender, route of infection, HCV viral genotype and serum viral load, body mass index (BMI), alcohol consumption before the first biopsy, serum ALT and serum glutamyl-transferase (GGT) levels were recorded for each patient.
Histological analysis
Liver biopsy specimens were fixed, paraffin-embedded, and routinely stained with haematoxylin-eosin and Masson’s trichrome and picrosirius red for collagen. For each liver biopsy specimen, stage of fibrosis (from 0 to 4) and grade of necro-inflammation including portal inflammation (from 0 to 4), periportal piecemeal necrosis (from 0 to 10) and intralobular inflammation (from 0 to 4) were established according to the Knodell score criteria. Worsening of the necro-inflammation (sum of portal inflammation, periportal piecemeal necrosis and intralobular inflammation scores) and fibrosis were defined by an increase of at least 2 and 1 points, respectively.
RNA quantification and procedure for HCV genotyping
Serum HCV RNA quantitative detection was performed using the RT-PCR method with a sensitivity limit of 100 copies/mL (Amplicor® Roche, Switzerland). Genotypes were identified using the INNO-LIPA HCV procedure (Innogenetics, Belgium).
Statistical analysis
SPSS software version 10.0 (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis. Quantitative variables were compared using Student’s t-test or non-parametric Mann-Whitney variance analysis (ANOVA). Qualitative variables were compared using the χ2 test or the Fischer test when necessary. Multivariate analysis was done using robust logistic regression. A two-tailed P value less than 0.05 was considered as significant.
RESULTS
Among our HCV-infected patient group, 410 patients had a first liver biopsy with a Knodell score ≤ 3. Only 76 patients, 34 males and 42 females, had at least a second liver biopsy and fulfilled the selection criteria. Their main characteristics are given in Table 1. Mean age at infection and at the first biopsy were 25 ± 9 years and 38 ± 9 years, respectively. Thirty-three patients (43.4%) had been contaminated by intravenous drug use and 28 (36.8%) by transfusions. Forty patients (55.6%) were infected with genotype 1 and 15 (20.8%) with genotype 3. The median duration of infection before the first biopsy was 13 (95% CI: 1-28) years and the median time between paired biopsies was 4 (95% CI: 2-16) years. The mean number of ALT level available between the 2 biopsies was 6 ± 3. During the follow-up period, 33 patients (43.4%) had normal serum ALT activity, 33 (43.4%) patients displayed occasional mild ALT increases (less than 2 times the upper normal limit) and 10 patients (13.2%) had constantly elevated ALT (> 3N). The mean daily alcohol consumption was 22.2 g/d (95% CI: 0-250). Mean BMI was 23.5 ± 3.1 for males and 22.0 ± 5.1 for females, without significant difference. Forty patients (55.6%) were infected with genotype 1; 9 (12.5%) with genotype 2; 15 (20.8%) with genotype 3; 6 (8.3%) with genotype 4 and 2 (2.8%) with genotype 5. Genotype was unknown for 4 patients. Viral load was low (under 350 000 UI/mL) for 33 patients (55%), medium (350 000 to 700 000 UI/mL) for 14 patients (23.3%) and high (more than 700 000 UI/mL) for 13 patients (21.7%). At the first biopsy, mean values for necro-inflammation and fibrosis were 1.75 ± 0.68 and 0.57 ± 0.5 respectively.
Table 1.
Demographic and clinical features of HCV patients with initially normal liver
| Demographic and clinical features | Data |
| Number of patients | 76 |
| Sex (M/F) | 34/42 |
| Age at the first biopsy (mean, yr) | 38 ± 9 |
| Age at Infection (mean, yr) | 25 ± 9 |
| BMI (M/F) | 23.5 ± 3.1/22 ± 5.1 |
| Route of infection:Transfusion | 28 (36.8%) |
| IVDU | 33 (43.4%) |
| Other or unknown | 15 (20%) |
| Genotype 1/2/3/4/5 | 40/9/15/6/2 |
| Infection duration, median (95% CI) | 13 (1-28) |
| Times between 2 biopsies, median (95% CI) | 4 (2-16) |
| Alcohol consumption (g/d, mean ± SD) | 22.2 ± 44 |
| ALT level between 2 biopsies | |
| Constantly normal | 42 (55.3%) |
| Normal or < 2 N | 31 (40.8%) |
| > 2 N | 3 (3.9%) |
| Viral load: | |
| Low (under 350 000 UI/mL) | 33 (55%) |
| Medium | 14 (23.3%) |
| High (more than 700 000 UI/mL) | 13 (21.7%) |
| Knodell score at the first liver biopsy | |
| Necroinflammatory index (mean ± SD) | 1.7 ± 0.7 |
| Fibrosis score (mean ± SD) | 0.6 ± 0.5 |
At the last biopsy, a significant increase in necro-inflammation (≥ 2 points) and fibrosis score (≥ 1 point) was observed in 38 (50%) and 26 patients (34%) respectively; 3 patients having a fibrosis equal to 3 and one equal to 4 (Figure 1). The mean difference in the necro-inflammation and fibrosis scores between the 2 biopsies was low: 1.79 ± 2.23 and 0.42 ± 0.77, respectively. Univariate analysis showed there was no difference between activity and fibrosis evolution according to the genotype, viral load or the infection duration. A higher fibrosis progression (1.00 ± 1.3) was observed in patients with BMI > 25 as compared to patients with BMI < 25 (0.28 ± 0.53) (P = 0.03). A significant progression in activity (mean = 1.7 ± 0.8 vs 0.4 ± 0.5) (P < 0.05) or fibrosis (mean = 0.9 ± 0.3 vs 0.0 ± 0.2) (P < 0.01) was observed in patients with elevated ALT as compared to patients with normal ALT. There was also a significant correlation between activity progression and fibrosis progression (P = 0.003).
Figure 1.
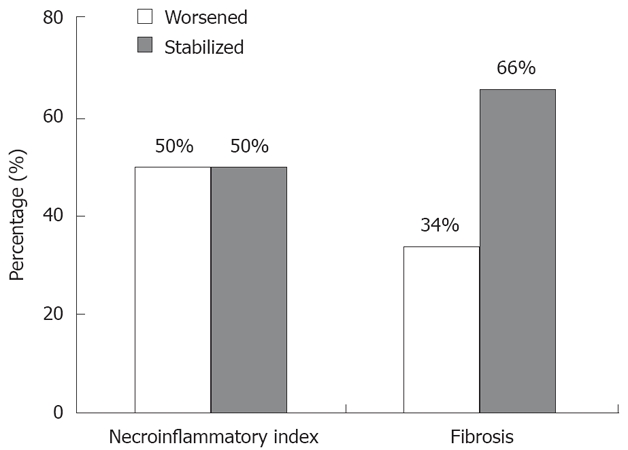
Pathological evolution. 38 patients (50%) had progression of necroinflammatory activity (progression ≥ 2) and 26 (34%) had progression of fibrosis (progression ≥ 1; 3 patients having a fibrosis score at 3 and one at 4).
By multivariate analysis, factors independently associated with liver fibrosis progression were an elevated ALT (RR = 7.5, CI = 1.4) (P = 0.02), BMI > 25 (RR = 4.9, CI = 1.2) (P = 0.03) and the interval between the 2 biopsies (RR = 1.8, CI = 1.3) (P = 0.001).
DISCUSSION
The long-term natural history of the so-called healthy carriers of HCV is not clear. Although ALT levels do not reflect the severity of the liver damage [11], patients with persistently normal ALT levels usually have a less severe disease, corresponding to a lower progression of fibrosis[8,12–15]. Nevertheless, some reports suggest the presence of significant fibrosis or cirrhosis in some of them[8,13,16,17]. Other reports underline the relationship between excess weight and hepatitis C-related fibrosis progression[18,19]. This study was designed to evaluate whether HCV-infected subjects with pathologically normal liver had any progression of liver damage after 4 years of follow-up. This might allow the screening of patients for which antiviral treatment would be helpful.
In our study, fibrosis progression only concerns one third of the patients. The short time between two biopsies cannot exclude a later worsening of liver fibrosis, which is probably slow, with a low risk of evolution to cirrhosis. Although ALT levels do not reflect the severity of the liver disease, elevated ALT was associated with liver fibrosis progression in this population with initially normal liver. Overweight, accordingly to the recent literature was also associated with fibrosis worsening[19]. Patients with elevated ALT or BMI may have more necro-inflammatory activity resulting in more pronounced fibrosis progression[19–21].
Our results suggest that ALT level follow-up is necessary whatever the histopathological results. In the subgroup of patients with elevated ALT or BMI, pathological follow-up seems to be useful and weight loss should be proposed. Our study suggests that liver fibrosis progression is correlated with time between biopsies, which probably make histopathological controls necessary. In patients with normal liver, normal ALT level and without co-morbidities such as excess weight, the time between biopsies should be longer than 5 years.
In our study, alcohol consumption was not correlated with fibrosis progression. We recorded the alcohol consumption before the first biopsy. We can suggest as a major hypothesis for this unusual result, that patients reduced their alcohol consumption after knowledge of their HCV status during follow up[22].
Antiviral therapy is usually not used in the so-called “asymptomatic HCV-carriers”[23,24]. We can differentiate in these “asymptomatic carriers” a sub-group which is at risk of fibrosis progression. The question is whether and when pegylated interferon and ribavirin should be a therapeutic option? Theoretically, if patients with normal liver tests do not really need treatment, therapy still can be proposed to interested patients with the same response rate as patients with elevated transaminases [25–29]. In addition, patients with a higher risk of liver fibrosis progression should be treated, particularly in case of genotype 2 or 3 infection[30].
In conclusion, this study confirms that the ‘‘HCV-healthy carrier’’ state does exist. Fibrosis does not worsen in two thirds of HCV-carriers without histopathological features after 4 years, supporting the concept that the natural history of chronic hepatitis in this group of subjects is characterized by a very slow or no progression. Antiviral therapy is not recommended in these patients with normal ALT or BMI under 25. Overweight, HCV-infected patient should be informed of the risk of liver fibrosis progression and the need of dietetic councils.
COMMENTS
Background
Chronic hepatitis C virus (HCV) infection cause liver damage, with a fibrotic scarring, which can progress to cirrhosis. The natural history of the infection varies among patients. For instance, in 10% of the case, the liver appears pathologically normal. Altogether, 10% to 40% of HCV infected patients harbor normal liver tests and the disease progresses very slowly as compared with patients with elevated liver tests. Until now, studies usually differentiate patients with or without liver test abnormalities but rarely patients with or without liver biopsy abnormalities.
Research frontiers
The goal of this work was to evaluate and differentiate patients for whom treatment will offer a better management of the disease. Among HCV infected patients without significant liver damage, one-third progress toward fibrosis. This work focuses on early detection of these patients with a view to treatment before fibrosis onset.
Innovations and breakthroughs
In two thirds of HCV infected patients without significant liver damage, there is no fibrosis progression. In these patients, fibrosis progression is associated with abnormal liver tests and elevated BMI.
Applications
These observations may be helpful to suggest if a patient should receive an antiviral therapy. A treatment should be counselled to the patients with abnormal liver test and elevated BMI.
Terminology
Liver fibrosis is the excessive accumulation of a scarred tissue that occurs in most types of chronic liver diseases. This fibrosis can progress to cirrhosis. The transaminases are a group of liver enzymes including alanine aminotransferase (ALT). Elevated transaminases can be an indicator of liver damage.
Peer review
Sobesky et al present an interesting study describing the features and development of individuals with chronic hepatitis C infection and almost normal histological findings. The paper is properly written.
Peer reviewer: Dr. Stefan Wirth, Professor, Children’s Hospital, Heusnerstt. 40, Wuppertal 42349, Germany
S- Editor Li DL L- Editor Lalor PF E- Editor Ma WH
References
- 1.Seymour CA. Asymptomatic infection with hepatitis C virus. BMJ. 1994;308:670–671. doi: 10.1136/bmj.308.6930.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everhart JE, Stolar M, Hoofnagle JH. Management of hepatitis C: a national survey of gastroenterologists and hepatologists. Hepatology. 1997;26:78S–82S. doi: 10.1002/hep.510260714. [DOI] [PubMed] [Google Scholar]
- 3.Okanoue T, Yasui K, Sakamoto S, Minami M, Nagao Y, Itoh Y, Kagawa K, Kashima K. Circulating HCV-RNA, HCV genotype, and liver histology in asymptomatic individuals reactive for anti-HCV antibody and their follow-up study. Liver. 1996;16:241–247. doi: 10.1111/j.1600-0676.1996.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 4.Collier JD, Woodall T, Wight DG, Shore S, Gimson AE, Alexander GJ. Predicting progressive hepatic fibrosis stage on subsequent liver biopsy in chronic hepatitis C virus infection. J Viral Hepat. 2005;12:74–80. doi: 10.1111/j.1365-2893.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 5.Mathurin P, Moussalli J, Cadranel JF, Thibault V, Charlotte F, Dumouchel P, Cazier A, Huraux JM, Devergie B, Vidaud M, et al. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868–872. doi: 10.1002/hep.510270333. [DOI] [PubMed] [Google Scholar]
- 6.Martinot-Peignoux M, Boyer N, Cazals-Hatem D, Pham BN, Gervais A, Le Breton V, Levy S, Degott C, Valla DC, Marcellin P. Prospective study on anti-hepatitis C virus-positive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology. 2001;34:1000–1005. doi: 10.1053/jhep.2001.28458. [DOI] [PubMed] [Google Scholar]
- 7.Persico M, Persico E, Suozzo R, Conte S, De Seta M, Coppola L, Palmentieri B, Sasso FC, Torella R. Natural history of hepatitis C virus carriers with persistently normal aminotransferase levels. Gastroenterology. 2000;118:760–764. doi: 10.1016/s0016-5085(00)70145-4. [DOI] [PubMed] [Google Scholar]
- 8.Pradat P, Alberti A, Poynard T, Esteban JI, Weiland O, Marcellin P, Badalamenti S, Trepo C. Predictive value of ALT levels for histologic findings in chronic hepatitis C: a European collaborative study. Hepatology. 2002;36:973–977. doi: 10.1053/jhep.2002.35530. [DOI] [PubMed] [Google Scholar]
- 9.Persico M, Perrotta S, Persico E, Terracciano L, Folgori A, Ruggeri L, Nicosia A, Vecchione R, Mura VL, Masarone M, et al. Hepatitis C virus carriers with persistently normal ALT levels: biological peculiarities and update of the natural history of liver disease at 10 years. J Viral Hepat. 2006;13:290–296. doi: 10.1111/j.1365-2893.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 10.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 11.Persico M, Romano M. Alanine aminotransferase measurements and histological disease in hepatitis C. Lancet. 1993;342:1369–1370. doi: 10.1016/0140-6736(93)92282-x. [DOI] [PubMed] [Google Scholar]
- 12.Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 13.Puoti C, Magrini A, Stati T, Rigato P, Montagnese F, Rossi P, Aldegheri L, Resta S. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology. 1997;26:1393–1398. doi: 10.1053/jhep.1997.v26.pm0009397976. [DOI] [PubMed] [Google Scholar]
- 14.Zarski JP, Mc Hutchison J, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, Truta B, Wright T, Gish R. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003;38:307–314. doi: 10.1016/s0168-8278(02)00387-2. [DOI] [PubMed] [Google Scholar]
- 15.Zylberberg H, Pol S, Thiers V, Chaix ML, Lagorce D, Brechot C, Nalpas B, Berthelot P. Significance of repeatedly normal aminotransferase activities in HCV-infected patients. J Clin Gastroenterol. 1999;29:71–75. doi: 10.1097/00004836-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Pasquale G, Sagnelli E, Coppola N, Scarano F, Scolastico C, Bellomo PF, Lettieri A, Piccinino F. Is liver biopsy necessary for hepatitis C virus carriers with persistently normal aminotransferase levels? Eur J Gastroenterol Hepatol. 2003;15:831–833. doi: 10.1097/01.meg.0000059137.68845.e2. [DOI] [PubMed] [Google Scholar]
- 17.Rumi MG, De Filippi F, Donato MF, Del Ninno E, Colombo M. Progressive hepatic fibrosis in healthy carriers of hepatitis C virus with a transaminase breakthrough. J Viral Hepat. 2002;9:71–74. doi: 10.1046/j.1365-2893.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 19.Perumalswami P, Kleiner DE, Lutchman G, Heller T, Borg B, Park Y, Liang TJ, Hoofnagle JH, Ghany MG. Steatosis and progression of fibrosis in untreated patients with chronic hepatitis C infection. Hepatology. 2006;43:780–787. doi: 10.1002/hep.21078. [DOI] [PubMed] [Google Scholar]
- 20.Bedossa P, Moucari R, Chelbi E, Asselah T, Paradis V, Vidaud M, Cazals-Hatem D, Boyer N, Valla D, Marcellin P. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007;46:380–387. doi: 10.1002/hep.21711. [DOI] [PubMed] [Google Scholar]
- 21.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Nalpas B, Martin S, Fontaine H, Fabbro-Peray P, Brechot C, Pol S. Impact of medical recommendations on alcohol consumption in HCV positive patients. J Hepatol. 2001;35:312–313. doi: 10.1016/s0168-8278(01)00114-3. [DOI] [PubMed] [Google Scholar]
- 23.Verslype C, Michielsen P, Adler M, Orlent H, Sprengers D, Delwaide J, D’heygere F, Langlet P, Brenard R, Colle I, et al. The management of patients with mild hepatitis C. Acta Gastroenterol Belg. 2005;68:314–318. [PubMed] [Google Scholar]
- 24.Sangiovanni A, Morales R, Spinzi G, Rumi M, Casiraghi A, Ceriani R, Colombo E, Fossati M, Prada A, Tavani E, et al. Interferon alfa treatment of HCV RNA carriers with persistently normal transaminase levels: a pilot randomized controlled study. Hepatology. 1998;27:853–856. doi: 10.1002/hep.510270330. [DOI] [PubMed] [Google Scholar]
- 25.Bini EJ, Mehandru S. Sustained virological response rates and health-related quality of life after interferon and ribavirin therapy in patients with chronic hepatitis C virus infection and persistently normal alanine aminotransferase levels. Aliment Pharmacol Ther. 2006;23:777–785. doi: 10.1111/j.1365-2036.2006.02819.x. [DOI] [PubMed] [Google Scholar]
- 26.Hasan F, Asker H, Al-Khalid J, Al-Mekhaizeem K, Al- Shamali M, Siddique I, Al-Nakib B. Interferon-alpha in combination with ribavirin for the treatment of chronic hepatitis C in patients with persistently normal aminotransferase levels. Digestion. 2002;65:127–130. doi: 10.1159/000057714. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson IM, Ahmed F, Russo MW, Lebovics E, Dieterich DT, Esposito SP, Bach N, Klion F, Tobias H, Antignano L, et al. Interferon alfa-2b [correction of alpha-2b]and ribavirin for patients with chronic hepatitis C and normal ALT. Am J Gastroenterol. 2004;99:1700–1705. doi: 10.1111/j.1572-0241.2004.30049.x. [DOI] [PubMed] [Google Scholar]
- 28.Rossini A, Ravaggi A, Biasi L, Agostinelli E, Bercich L, Gazzola GB, Callea F, Radaeli E, Cariani E. Virological response to interferon treatment in hepatitis C virus carriers with normal aminotransferase levels and chronic hepatitis. Hepatology. 1997;26:1012–1017. doi: 10.1002/hep.510260432. [DOI] [PubMed] [Google Scholar]
- 29.Shiffman ML, Stewart CA, Hofmann CM, Contos MJ, Luketic VA, Sterling RK, Sanyal AJ. Chronic infection with hepatitis C virus in patients with elevated or persistently normal serum alanine aminotransferase levels: comparison of hepatic histology and response to interferon therapy. J Infect Dis. 2000;182:1595–1601. doi: 10.1086/317612. [DOI] [PubMed] [Google Scholar]
- 30.Bacon BR. Treatment of patients with hepatitis C and normal serum aminotransferase levels. Hepatology. 2002;36:S179–S184. doi: 10.1053/jhep.2002.36386. [DOI] [PubMed] [Google Scholar]


