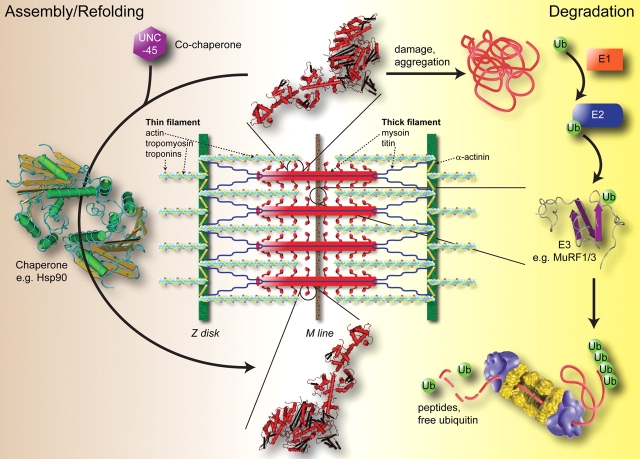
Figure 1.
Sarcomere-specific chaperones and ubiquitin ligases are necessary for the assembly and degradation of sarcomere proteins and constitute the protein quality control system in the heart. The protein quality control of the sarcomere involves the continuous assembly (left side) and degradation (right side) of specific sarcomere proteins. In this example, the co-chaperones UNC-45 and Hsp90 are required for the assembly of myosin. This is balanced by the specific ubiquitination and degradation of proteins by the ubiquitin proteasome system. This involves specific ubiquitin ligases (designated E3) that place poly-ubiquitin tails on targets for degradation by the 26S proteasome. In this example, both MuRF1 and MuRF3 have shown to specifically ubiquitinate and degrade myosin in a proteasome-dependent manner. In the heart, this dynamic process of protein quality control occurs amid continuous use in order to maintain the fundamental construct necessary for contractility. Proteasome graphic courtesy of the U.S. Department of Energy Genome Programs (http://genomics.energy.gov).