Abstract
BACKGROUND
Spontaneous dizygotic (DZ) twinning in humans is under genetic control. In sheep, heterozygous loss of function mutations in bone morphogenetic protein 15 (BMP15) increase ovulation and hence twinning rates.
METHODS
To investigate the role of BMP15 in human twinning, we typed 14 common variants, 4 rare novel variants initially detected by sequencing 279 mothers of DZ twins (MODZT) and 17 variants previously associated with premature ovarian failure (POF) in 933 DZ twinning families. We also typed five additional POF associated GDF9 variants.
RESULTS
There was some evidence for association between DZ twinning and a common intronic BMP15 variant (rs3897937), but this was not significant after correction for multiple testing. Three of the four novel variants (p.Pro174Ser, p.Ala311Thr and p.Arg392Thr) occurred in 1–5 MODZT but were not detected in 1512 controls. We also detected three POF associated mutations in both BMP15 and GDF9 at low frequencies in MODZT and controls.
CONCLUSIONS
We conclude that neither rare nor common BMP15 variants play a significant role in the variation in human DZ twinning.
Keywords: dizygotic twinning, BMP15, variation, genetic association, primary ovarian failure
Introduction
The transforming growth factor signalling pathway within the ovary is critical for the regulation of ovarian function, ovulation rate and fertility (Moore et al., 2004; Shimasaki et al., 2004). Two genes in this pathway are growth differentiation factor 9 (GDF9) on chromosome 5 and bone morphogenetic protein 15 (BMP15) on the X chromosome. Loss of function of Gdf9 in female mice blocks folliculogenesis during early follicle development and leads to infertility (McGrath et al., 1995). In sheep, the effects of mutations in both GDF9 and BMP15 are sensitive to copy number. Heterozygous mutations in both genes increase the frequency of twins and higher order multiples, whereas homozygous loss of function mutations results in ovarian dysgenesis and infertility (Galloway et al., 2000; Hanrahan et al., 2004).
This pathway is also essential for human fertility (Di Pasquale et al., 2004). Two sisters with hypergonadotrophic ovarian failure due to ovarian dysgenesis were found to carry a non-conservative amino acid substitution in the pro region of BMP15 (p.Tyr235Cys) which acts in a dominant negative fashion by altering BMP15 processing (Di Pasquale et al., 2004). Rare variants in both BMP15 and GDF9 contribute to premature ovarian failure (POF) (Di Pasquale et al., 2004, 2006; Dixit et al., 2005, 2006; Laissue et al., 2006). Additionally, we found that some mothers of spontaneous dizygotic (DZ) twins (MODZT) carry rare deletions and missense mutations in the coding region of GDF9 that are significantly associated with twinning (Montgomery et al., 2004; Palmer et al., 2006).
BMP15 is expressed in oocytes in several mammalian species (Laitinen et al., 1998; Galloway et al., 2000; Otsuka et al., 2000). The protein has six conserved cysteine residues characteristic of the TGFβ superfamily, but lacks the additional cysteine residue that strengthens dimerization in other members of the bone morphogenetic protein family (Vitt et al., 2001). BMP15 stimulates granulosa cell proliferation (Otsuka et al., 2000), inhibits follicle-stimulating hormone receptor (FSHR) expression (Otsuka et al., 2001) and stimulates KIT ligand expression (Otsuka and Shimasaki, 2002).
Five different heterozygous mutations in the coding region of BMP15 in different lines of sheep increase ovulation rate and litter size (McNatty et al., 2004). It is unknown if variants in BMP15 contribute to variation in human twinning. The human BMP15 sequence has a number of variants that alter the predicted protein sequence, including rs41308602 (c.308A>G) located in the second position of codon 103 of the preproprotein that changes the amino acid from an asparagine to a serine. We hypothesized that variation in BMP15 may contribute to the variation in human twinning and genotyped common variants identified from the literature and public databases in families of MODZT and controls. We also searched for rare variants by conducting a mutation screen of the coding region of the BMP15 gene in a subset of MODZT and typed these novel variants in additional families to determine whether these are associated with DZ twinning. Further, we typed rare BMP15 and GDF9 variants previously reported in patients with POF to determine whether these POF associated variants might contribute to the risk for DZ twinning.
Materials and Methods
Experimental subjects
Study subjects were Caucasians recruited from 933 families with a history of DZ twinning (770 families from Australia and New Zealand and 163 families from the Netherlands) with 3450 individuals available for genotyping, including 1693 MODZT. We recruited 406 families with two or more sisters who had given birth to spontaneous DZ twins (Duffy et al., 2000) and also 527 families with a single case where at least one-third degree female relative had spontaneous DZ twins. Samples were also obtained from parents of MODZT where available and from additional sibs. MODZT and their families were identified through records from our genetic epidemiology studies using twins and their families in Australia (Lewis et al., 1996), through organizations for mothers of twins in Australia and New Zealand, and through appeals in the media in both countries. In the Netherlands, ascertainment was population-based through community records as part of a systematic recruitment to the Netherlands Twin Register (Meulemans et al., 1996; Boomsma et al., 2002). Mothers were explicitly asked about fertility treatments and all such cases were excluded.
Genetic investigation was extended to a population-based control group of 1512 Caucasian females and males (unselected for twinning history), selected at random from the electoral roll in Australia. Study protocols were reviewed and approved by the Human Research Ethics Committee of the Queensland Institute of Medical Research and the Ethics Committee of the Vrije Universiteit Hospital. Participation was voluntary and each participant gave written informed consent.
Genomic DNA was extracted (Miller et al., 1988) from peripheral venous blood samples. Zygosity of the mothers' twin offspring was determined from differences in sex, eye colour or hair colour and, in equivocal cases, by typing nine independent microsatellite markers (AmpFLSTR® Profiler Plus™, Applied Biosystems, Foster City, CA, USA). The probability of dizygosity given concordance of all markers in the panel was <10−4.
Denaturing high performance liquid chromatography (DHPLC) analysis
PCR fragments covering the entire coding sequence and intron–exon junctions of the BMP15 gene were analysed in 279 MODZT, where one sister was drawn from each of 279 affected sister pair families. PCR reactions were performed in 20 µl volumes containing 15 ng of DNA, 1× PCR buffer, 16 pmol of each primer, 1.5 mM MgCl2, 200 µM dNTPs and 1 U Amplitaq Gold (Applied Biosystems). Prior to DHPLC, amplicons were denatured at 95°C for 5 min and cooled to 60°C, dropping by 5°C increments with 4 min at each temperature. PCR products were then injected into a Varian Helix System (Varian, Walnut Creek, CA, USA) and eluted within a linear acetonitrile gradient consisting of buffer A [0.1 M triethylammonium acetate (TEAA) and 0.1 mM EDTA] and buffer B (0.1 M TEAA, 0.1 mM EDTA and 25% acetonitrile) with a flow rate of 0.45 ml/min. The buffer B gradient was 45–50% (0–0.5 min), 50–68% (0.5–6 min), 68% (6–7 min) and 68–45% (7–8 min). DHPLC was carried out, on a fragment specific basis, at the optimal temperature as determined by the Stanford Genome Technology Centre DHPLC melt program (http://insertion.stanford.edu/melt.html). Analyses were performed using the Star Workstation version 5 (Varian). The appearance of additional peaks was interpreted as indicative of a mismatch in the PCR fragment. For these samples, new PCR products amplifying the entire exon of interest were generated and purified by Microcon-PCR Centrifugal Filter devices (Millipore, Billerica, MA, USA). BigDye® Terminator v3.1 terminator premix (Applied Biosystems) was used for cycle sequencing with purified PCR products analysed with a capillary based genetic analyser.
SNP genotyping
All common, novel and POF associated SNPs were typed in the 3450 individuals from 933 twinning families and 1512 controls using the Sequenom™ iPLEX™ protocol. Genotyping assays were designed using MassARRAY Assay Design software (Sequenom Inc., San Diego, CA, USA). The 2.5 µl PCR reactions were performed in standard 384-well plates using 10 ng genomic DNA, 0.5 U Taq polymerase (HotStarTaq, Qiagen, Valencia, CA, USA), 500 µmol of each dNTP and 100 nmol of each PCR primer. PCR thermal cycling in an ABI-9700 instrument was 15 min at 94°C, followed by 45 cycles of 20 s at 94°C, 30 s at 56°C, 60 s at 72°C. The completed PCR reactions were then incubated with 0.15 U shrimp alkaline phosphatase for 30 min at 37°C followed by inactivation for 5 min at 85°C. After adjusting the concentrations of extension primers to equilibrate signal-to-noise ratios, the post-PCR primer extension reaction of the iPLEX assay was performed in a final 5 µl extension reaction containing 0.1 µl of termination mix, 0.02 µl of DNA polymerase (Sequenom Inc.) and 600–1200 nM extension primers. A two-step 200 short-cycles program was used for the iPLEX extension reaction: initial denaturation was 30 s at 94°C followed by five cycles of 5 s at 52°C and 5 s at 80°C. An additional 40 annealing and extension cycles were then looped back to 5 s at 94°C, 5 s at 52°C and 5 s at 80°C. A final extension was carried out at 72°C for 3 min and then the sample was cooled to 20°C. The iPLEX reaction products were desalted by diluting samples with 15 µl of water and 3 µl of resin to optimize mass spectrometric analysis. Products were spotted on a SpectroChip (Sequenom Inc.), and processed and analysed in a Compact Mass Spectrometer using MassARRAY Workstation (version 3.3) software (Sequenom Inc.).
Statistical analysis
The program Sib-pair (http://www.qimr.edu.au/davidD/sib-pair.html) was used to calculate preliminary allele and genotype frequencies. Since variants were genotyped in families, the case–control comparisons of allele frequencies to test association allowing for the family nature of the data were carried out using the program MENDEL 7.0 (Lange et al., 2001). To make the comparison as clear as possible, a case was defined as an MODZT, whereas a control was a member (male or female) of our population-based sample; other relatives of cases were treated as having unknown phenotypes. We fitted allelic association models (i.e. assuming multiplicative effects on penetrance in females), and so could utilize male controls (remembering that BMP15 is X-linked). For association analysis of rare variants discovered via sequencing (where only cases were sequenced), we carried out ascertainment correction (conditioning the family likelihood on that of the sequenced proband). Reconstruction of haplotypes consisting of four common SNPs surrounding and within exon 1, and the analysis of haplotype frequencies in MODZT and controls were performed using MENDEL 7.0.
To predict the functional significance of missense mutations, we constructed a multiple sequence alignment for mammalian BMP15 protein sequences for human (NP_005439.1), macaque (XP_001083980), chimpanzee (XP_529247), sheep (Q9MZE2), cow (NP_001026922), pig (NP_001005155), mouse (NP_033887) and rat (NP_067702) sequences and compared the amino acid substitutions using the Align-GVGD program (Mathe et al., 2006) available at http://agvgd.iarc.fr. The program uses an extension of the Grantham difference and compares the amino acid substitutions taking into account composition, polarity and volume of amino acid substitutions within the context of a multiple sequence alignment for the protein. Amino acid changes are classified as to their likelihood of interfering with protein function on a scale from class C65 (most likely) to C0 (least likely).
Results
Common SNPs
We typed 14 common SNPs located across the BMP15 locus identified from public databases (all SNPs with rs designations) and the literature (c.-673C>T, Moron et al., 2006; Fig. 1). Two SNPs (rs6614369 and rs6614608) were monomorphic in our samples and were omitted from all analyses. Genotype data for the remaining 12 common SNPs were in Hardy–Weinberg equilibrium, and overall minor allele frequencies ranged from 0.001 to 0.327 (Table I). The analysis of allelic association for individual SNPs identified some evidence of association between the DZ twinning phenotype and the intronic SNP rs3897937 (IVS1+905G>A) with a significantly higher frequency of the A allele in MODZT than in controls (0.325 versus 0.294, P = 0.010, Table II). A SNP in the promoter region (rs3810682; c.-9C>G) also showed some evidence of association. However, the differences in allele frequencies between MODZT and controls were small and the effects were not significant after correcting for multiple testing of all SNPs.
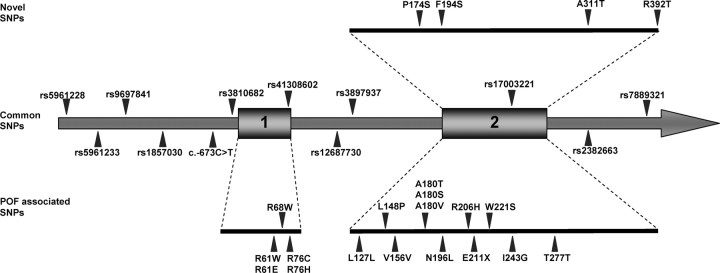
Figure 1:
Schematic representation of the genomic structure of the BMP15 gene showing the locations of the SNPs genotyped in our DZ twinning families, including 12 common SNPs, 4 rare variants identified in mutation screening of MODZT and 17 POF associated variants. Common SNPs that were not polymorphic in our samples are not shown, and only the exons are drawn to scale.
Table I.
Common SNPs at the BMP15 locus genotyped in 933 twinning families and 1512 controls.
| SNP | Position* | Location | Amino acid change | Change (transcribed strand) | Minor allele frequency |
|---|---|---|---|---|---|
| rs5961228 | 50656568 | 5′ genomic | — | C>T | 0.135 |
| rs5961233 | 50663804 | 5′ genomic | — | C>A | 0.101 |
| rs9697841 | 50664677 | 5′ genomic | — | T>G | 0.061 |
| rs1857030 | 50667633 | 5′ genomic | — | T>A | 0.061 |
| c.-673C>T | 50671179 | 5′ genomic | — | C>T | 0.240 |
| rs3810682 | 50670515 | 5′ UTR | — | C>G | 0.232 |
| rs41308602 | 50670830 | Exon 1 | Asn103Ser | c.308A>G | 0.086 |
| rs12687730 | 50671338 | Intron 1 | — | T>C | 0.001 |
| rs3897937 | 50671756 | Intron 1 | — | T>C | 0.294 |
| rs17003221 | 50676020 | Exon 2 | Ser248Ser | c.852C>T | 0.005 |
| rs2382663 | 50679039 | 3′ genomic | — | G>A | 0.327 |
| rs7889321 | 50692668 | 3′ genomic | — | A>G | 0.038 |
*Genomic position according to NCBI Build 36.1.
Table II.
Allelic association between dizygotic twinning and common SNPs at the BMP15 locus.
| SNP | N genotyped | Minor allele frequency |
Allelic χ2 | P-value1 | |
|---|---|---|---|---|---|
| MODZT N = 1693 | Controls N = 1512 | ||||
| rs5961228 | 4648 | 0.137 | 0.141 | 2.032 | 0.154 |
| rs5961233 | 4633 | 0.102 | 0.103 | 0.002 | 0.952 |
| rs9697841 | 4628 | 0.065 | 0.059 | 1.250 | 0.289 |
| rs1857030 | 4637 | 0.064 | 0.057 | 1.161 | 0.281 |
| c.-673C>T | 4680 | 0.250 | 0.230 | 2.390 | 0.122 |
| rs3810682 | 4534 | 0.255 | 0.232 | 3.503 | 0.061 |
| rs41308602 | 4596 | 0.088 | 0.086 | 0.414 | 0.519 |
| rs12687730 | 4613 | 0.0001 | 0.002 | 2.628 | 0.105 |
| rs3897937 | 4581 | 0.325 | 0.294 | 6.705 | 0.010 |
| rs17003221 | 4649 | 0.004 | 0.005 | 1.971 | 0.160 |
| rs2382663 | 4563 | 0.319 | 0.328 | 0.614 | 0.433 |
| rs7889321 | 4648 | 0.038 | 0.038 | 0.421 | 0.516 |
1Asymptotic P-values were estimated allowing for the family nature of the data using the measured genotype approach implemented in MENDEL 7.0 (Lange et al., 2001).
We estimated the frequencies of haplotypes including four common SNPs located around and within exon 1 of BMP15, including rs3810682 and rs3897937, following recent evidence that a haplotype comprising these SNPs is associated with the production of increased numbers of follicles (≥12) during ovarian stimulation (Moron et al., 2006). In our samples only four haplotypes had frequencies >5% in both MODZT and controls (Table III). One common haplotype accounted for 67% of BMP15 chromosomes among our MODZT, with the two most frequent haplotypes accounting for >82% of chromosomes. There were no differences in the frequencies of haplotypes between MODZT and controls (Table III).
Table III.
Frequencies of observed haplotypes and odds ratios for MODZT (cases) and controls for four common BMP15 SNPs.
| Haplotype c.-673C>T–rs3810682–rs41308602–rs3897937 | Frequency MODZT | Frequency controls | Odds ratio |
|---|---|---|---|
| C C A A | 0.668 | 0.692 | 1.000 |
| T G A G* | 0.155 | 0.136 | 0.846 |
| T G G G* | 0.077 | 0.092 | 1.153 |
| C C A G | 0.063 | 0.058 | 0.888 |
| Rare haplotypes | 0.034 | 0.021 | 0.704 |
Haplotypes with frequencies ≤0.01 in both MODZT and controls were collapsed and analysed as a single group of ‘rare haplotypes’.
*A haplotype associated with the production of high numbers of follicles during assisted reproduction by Moron et al. (2006), TGG, is composed of markers c.-673C>T–rs3810682–rs3897937, incorporating either allele of rs41308602.
Novel variants in MODZT
A search for rare variants in the BMP15 gene in 279 MODZT identified four rare missense alterations resulting in putative changes in the amino acid sequence. Two variants (c.520C>T, p.Pro174Ser and c.581T>C, p.Phe194Ser) were located in the pro-region of BMP15 and two variants (c.931G>A, p.Ala311Thr and c.1175G>C, p.Arg392Thr) in the mature protein region. The p.Arg392Thr variant was predicted as ‘most likely’ to interfere with protein function (Align-GVGD class C65), p.Phe194Ser and p.Ala311Thr as slightly less likely to affect protein function (class C55, one class below C65), whereas p.Pro174Ser is predicted to have no effect (class C0).
These variants were then typed in all samples (Table IV). The p.Phe194Ser variant had a higher frequency in controls than in MODZT (0.003 versus 0.001, P = 0.054). The other three variants were seen in twinning families but not controls; the p.Pro174Ser variant in additional members of the family in which it was first found, the p.Ala311Thr variant in members of the original and one additional family and the p.Arg392Thr variant only in the sample in which it was first detected. Corrected allele frequencies taking ascertainment bias into account were not significantly different between MODZT and controls (Table IV).
Table IV.
BMP15 variants identified by DHPLC analysis of 279 MODZT.
| BMP15 variant | Protein variant | MODZT |
Controls |
P-value | ||
|---|---|---|---|---|---|---|
| Carriers | Allele freq | Carriers | Allele freq | |||
| c.520C>T | Pro174Ser | 2 | 0.0005 | — | — | 0.112 |
| c.581T>C | Phe194Ser | 5 | 0.0012 | 11 | 0.0036 | 0.054 |
| c.931G>A | Ala311Thr | 3 | 0.0008 | — | — | 0.066 |
| c.1175G>C | Arg392Thr | 1 | 0.0002 | — | — | 0.295 |
| Any of the above | — | 11 | 0.0032 | 11 | 0.0036 | 0.168 |
Numbers of carriers and minor allele frequencies were determined by Sequenom MALDI-TOF SNP analysis of 933 DZ twinning families with 1693 MODZT and 1512 controls. The P-values were calculated for the likelihood ratio test (MENDEL binomial link measured genotype model) testing for effect of the variant on the likelihood of having twins under a multiplicative model.
POF associated variants in MODZT
Seventeen variants in the coding regions of BMP15 and six in GDF9 that have been previously reported in POF patients were also typed in our 933 twinning families and 1512 controls (Table V). Three POF associated BMP15 variants were detected in our samples, at approximately similar minor allele frequencies for both MODZT and controls (p.Arg68Trp, 0.0003 versus 0.002, P = 0.303; p.Ala180Thr, 0.014 versus 0.016, P = 0.253; p. Leu263_Arg264insLeu, 0.005 versus 0.005, P = 0.406).
Table V.
Summary of mutations identified in women with premature ovarian failure (POF) in the GDF9 and BMP15 genes.
| Gene and sequence change | Amino Acid Change | Initial study population | No. POF mutations | References | No. of Carriers |
|
|---|---|---|---|---|---|---|
| MODZT | Controls | |||||
| GDF9 | ||||||
| c.-8C>T | — | Indian | 2/195 | Dixit et al. (2005) | — | 1 |
| c.199A>C | Lys67Glu | Indian | 5/195 | Dixit et al. (2005) | — | 1 |
| c.205C>T | Ser70Ser | Indian | 1/195 | Dixit et al. (2005) | 1 | — |
| c.307C>T | Pro103Ser | Caucasian | 1/61 | Kovanci et al. (2007) | 30* | 13* |
| c.646G>A | Val216Met | Indian | 2/195 | Dixit et al. (2005) | — | — |
| c.1353C>T | Cys461Cys | Indian | 3/195 | Dixit et al. (2005) | — | — |
| BMP15 | ||||||
| c.181C>T | Arg61Trp | Indian | 2/202 | Dixit et al. (2006) | — | — |
| c.182G>A | Arg61Glu | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.202C>T | Arg68Trp | Italian | 1/166 | Di Pasquale et al. (2006) | 2 | 3 |
| c.226C>T | Arg76Cys | Indian | 5/202 | Dixit et al. (2006) | — | — |
| c.227G>A | Arg76His | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.381A>G | Leu127Leu | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.443T>C | Leu148Pro | Mixed origins | 1/203 | Laissue et al. (2006) | — | — |
| African-American | 2/14 | Di Pasquale et al. (2006) | ||||
| c.468G>A | Val156Val | Mixed origins | 1/203 | Laissue et al. (2006) | — | — |
| c.538G>A | Ala180Thr | Italian | 5/166 | Di Pasquale et al. (2006) | 46 | 32 |
| Indian | 3/202 | Dixit et al. (2006) | ||||
| Mixed origins | 2/203 | Laissue et al. (2006) | ||||
| c.538G>T(+)c.539C>T | Ala180Phe/Ser+Val | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.588T>A | Asn196Lys | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.617G>A | Arg206His | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.631C>T | Glu211X | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.661T>C | Trp221Arg | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.727A>G | Ile243Gly | Indian | 1/202 | Dixit et al. (2006) | — | — |
| c.788_789insTCT | Leu263_Arg264insLeu | Indian | 9/202 | Dixit et al. (2006) | 16 | 12 |
| c.831T>C | Thr277Thr | Mixed origins | 1/203 | Laissue et al. (2006) | — | — |
SNPs were screened in 933 DZ twinning families (including 1693 MODZT) and 1512 controls.
*Found previously in this cohort, Palmer et al. (2006).
Three POF associated GDF9 variants were each detected in either a single control (c.-8C>T and p.Lys67Glu) or MODZT (p.Ser70Ser; Table V). Previously, the p.Pro103Ser variant had been found in this cohort at a higher frequency among our MODZT than among controls (Palmer et al., 2006; Table V).
Discussion
BMP15 is a strong candidate gene likely to contribute to the variation in human twinning. The gene is specifically expressed in the oocytes of developing follicles (Laitinen et al., 1998; Galloway et al., 2000; Otsuka et al., 2000) and several loss of function mutations in sheep increase ovulation rates and litter sizes in heterozygous carriers (Galloway et al., 2000; Hanrahan et al., 2004). To test for association between BMP15 variants and human twinning, we typed a total of 35 common, rare and novel BMP15 SNPs in our DZ twinning families. The frequency of the C allele of one intronic SNP (rs3897937) was higher in MODZT than in controls, although this was not significant after accounting for multiple testing. The analysis of the SNPs across the BMP15 locus showed strong linkage disequilibrium, with two haplotypes accounting for 82% of chromosomes in MODZT. There were no significant associations between haplotype frequencies and the DZ twinning phenotype.
We previously identified rare mutations in the GDF9 gene including both insertion/deletion and missense mutations with higher frequencies in MODZT compared with controls (Montgomery et al., 2004; Palmer et al., 2006). We carried out a similar screen of BMP15 in 279 probands from our most twin dense families. We found no insertion/deletion mutations, but did identify four rare missense BMP15 mutations in our MODZT. One of these (p.Phe194Ser) was more frequent in controls than in MODZT, and hence can probably be considered a rare polymorphism. The other three variants were seen only in twinning families. However, the variant most likely to affect protein function, p.Arg392Thr, was carried by one MODZT but not by her sister, also an MODZT. Conversely, p.Ala311Thr and p.Pro174Ser were detected in both MODZT and their ‘unaffected’ sisters. It is possible that these variants are twinning associated alleles with reduced penetrance; but although both are predicted to interfere to some degree with protein function, whether or not they actually have an effect is unknown. Additionally, the carrier status of all MODZT for the p.Ala311Thr variant within one of the two families in which it was detected cannot be determined due to the incomplete sampling of this very large extended pedigree. Taken together, our results suggest that the contribution of rare variants in BMP15 to the variability in human twinning would be small.
There are species differences in the actions of BMP15. Loss of function mutations in humans and sheep has dramatic consequences on follicle development. A mutation at codon 235 in the preproregion of human BMP15 associated with hypergonadotrophic ovarian failure in two sisters with streak ovaries (Di Pasquale et al., 2004) acts in a dominant negative fashion, abolishing the effects of wild-type BMP15 in stimulating the growth of granulosa cells (Di Pasquale et al., 2004). In contrast, the targeted deletion of the second exon of the Bmp15 gene in mice has limited effects on folliculogenesis (Yan et al., 2001). Female Bmp15 knockout mice are subfertile due to defects in the ovulation process and the ability of oocytes to develop into normal embryos (Liao et al., 2004). The differences between these species may reflect differences in the relative importance of BMP15 and GDF9 in regulating events of folliculogenesis (Liao et al., 2004) or may reflect the nature of the specific mutations. Studies with a recombinant human BMP15 p.Ile31Asp substitution (Liao et al., 2003), which mimics the p.Val31Asp variant in the Inverdale strain of sheep (Galloway et al., 2000), show that the variant form of the protein interferes with proteolytic processing of both wild-type BMP15 and also GDF9. The p.Tyr235Cys mutation in human BMP15 associated with hypergonadotrophic ovarian failure (Di Pasquale et al., 2004) also interferes with the processing and secretion of bioactive proteins.
Our data suggest variation in human BMP15 plays at best a very minor role in human DZ twinning. This contrasts with evidence for sheep where at least five different BMP15 mutations are associated with high ovulation rates and litter sizes in different flocks, where they have attained high frequencies due to artificial selection or genetic drift (McNatty et al., 2004). These variants have been identified in prolific breeds of sheep, and to date there has been no systematic screen for variants in either BMP15 or GDF9 in the wider sheep population to estimate the frequency of rare variants or the relative importance of variation in these two growth factors influencing ovulation rate. We have earlier shown that rare variants in human GDF9, including both insertion/deletion and missense mutations, account for ∼2.4% of the attributable risk for twins (Palmer et al., 2006). Taken together, our results suggest GDF9 plays a more important role than BMP15 in the regulation of twinning in humans (Montgomery et al., 2004; Palmer et al., 2006). Associations between DZ twinning and variants in other genes including FMR (Vianna-Morgante, 1999; Marozzi et al., 2000) and SERPINA1 (Clark and Martin, 1982; Boomsma et al., 1992) have been reported, but none have been replicated in large samples.
Four of the common BMP15 variants analysed here have recently been associated with high response to treatment with recombinant follicle-stimulating hormone (FSH) during assisted reproduction (Moron et al., 2006). Minor alleles for variants c.-673C>T plus rs3810682 (the two markers are in almost complete linkage disequilibrium) and rs3897937, as well as the resulting ‘TGG’ haplotype, were significantly over-represented among 35 high FSH responders (producing ≥12 follicles), and particularly in the subset of 11 high responders who developed ovarian hyperstimulation syndrome. Although the numbers of women involved were small (11.4% and 3.6% of the women tested, respectively), this suggests a role for BMP15 variants in the control of follicle numbers in response to high exogenous FSH concentrations, possibly through a functional variant linked to the TGG haplotype (Moron et al., 2006). Raised FSH concentrations during the follicular phase have been documented in MODZT (Nylander, 1974; Martin et al., 1984; Lambalk et al., 1998), and it may be that with this haplotypic background BMP15 also influences follicle number in response to high levels of endogenous FSH. However, we found no evidence that the frequency of the TGG haplotype was increased in MODZT.
Conversely, a number of recent studies identified variants in both BMP15 and GDF9 associated with POF (Dixit et al., 2005, 2006; Di Pasquale et al., 2006; Laissue et al., 2006). MODZT reach menopause significantly earlier than mothers of monozygotic twins, with the difference resulting from some MODZT reaching menopause before age 40 (Martin et al., 1997). This small increase in the frequency of POF in MODZT could be explained by mutations in GDF9 and/or BMP15 influencing both aspects of ovarian function. To test this hypothesis, 23 GDF9 and BMP15 variants previously associated with POF were typed in our 933 twinning families. We have earlier shown that the GDF9 variant p.Pro103Ser, also reported in one woman with POF (Kovanci et al., 2007), is significantly associated with twinning (Palmer et al., 2006). We detected three additional POF associated variants in each gene in our MODZT and/or controls—BMP15 variants p.Arg68Trp (Di Pasquale et al., 2006), p.Ala180Thr (Di Pasquale et al., 2006; Dixit et al., 2006; Laissue et al., 2006) and p.Leu263_Arg264insLeu (Dixit et al., 2006) and GDF9 variants c.-8C>T, p.Lys67Glu and p.Ser70Ser (Dixit et al., 2005). In previous reports, the p.Leu263_Arg264insLeu was associated with POF in one study (Dixit et al., 2006), but not in two others (Di Pasquale et al., 2006; Laissue et al., 2006). This and the other variants we detected may be rare polymorphisms rather than mutations, and further studies are now required to confirm whether these variants are in fact associated with POF.
The association of the GDF9 p.Pro103Ser variant with both twinning (Palmer et al., 2006) and POF (Kovanci et al., 2007) might, however, support the hypothesis that earlier menopause in mothers of twins and the twinning phenotype are related to mutations in GDF9 and that other mutations/genes causing POF may also be candidates for twinning. We found no globally significant association between twinning and any BMP15 variant or haplotype, but the possibility that multiple variants might affect either POF or twinning is intriguing. Further studies will be required to determine if such differences reflect the rare frequencies of these variants in each condition or whether variants can influence POF and twinning independently.
In conclusion, we found no evidence that either rare or common variants in BMP15 play any significant role in the variation in human DZ twinning. We cannot entirely rule out weak effects or epistatic interactions with other variants, but very large studies will be required to examine these effects. We identified rare variants in BMP15 in MODZT, but the frequencies of these were very low. There is evidence for an association between early menopause and twinning and although most POF variants were not found in MODZT, the GDF9 p.Pro103Ser variant associated with twinning (Palmer et al., 2006) has been reported in one POF patient (Kovanci et al., 2007). Further studies should examine the relationships between twinning and POF.
Funding
This study was supported by grants to GWM from the National Institute of Child Health and Human Development (HD042157) and National Health and Medical Research Council of Australia (159100 and 339446) and by the Cooperative Centre for the Discovery of Genes for Common Human Disease.
Acknowledgements
We thank Barbara Haddon and Alison MacKenzie for coordination of recruitment. We also thank the Multiple Birth Associations of Australia (AMBA) and New Zealand (NZAMBA) for assistance with recruitment and the mothers of twins and their families for participation in the research.
References
- Boomsma DI, Frants RR, Bank RA, Martin NG. Protease inhibitor (Pi) locus, fertility and twinning. Hum Genet. 1992;89:329–332. doi: 10.1007/BF00220552. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Vink JM, van Beijsterveldt TC, de Geus EJ, Beem AL, Mulder EJ, Derks EM, Riese H, Willemsen GA, Bartels M, et al. Netherlands Twin Register: a focus on longitudinal research. Twin Res. 2002;5:401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- Clark P, Martin NG. An excess of the Pi S allele in dizygotic twins and their mothers. Hum Genet. 1982;61:171–174. doi: 10.1007/BF00274213. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M, et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91:1976–1979. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. doi: 10.1007/s00439-006-0150-0. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Montgomery GW, Hall J, Mayne C, Healey SC, Brown J, Boomsma DI, Martin NG. Human twinning is not linked to the region of chromosome 4 synthetic with the sheep twinning gene FecB. Am J Med Genet. 2000;100:182–186. doi: 10.1002/ajmg.1255. [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta S, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsannt P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril. 2007;87:143–146. doi: 10.1016/j.fertnstert.2006.05.079. [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, et al. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol. 2006;154:739–744. doi: 10.1530/eje.1.02135. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech Dev. 1998;78:135–140. doi: 10.1016/s0925-4773(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Lambalk CB, Boomsma DI, De Boer L, De Koning CH, Schoute E, Popp-Snijders C, Schoemaker J. Increased levels and pulsatility of follicle-stimulating hormone in mothers of hereditary dizygotic twins. J Clin Endocrinol Metab. 1998;83:481–486. doi: 10.1210/jcem.83.2.4552. [DOI] [PubMed] [Google Scholar]
- Lange K, Cantor R, Horvath S, Perola M, Sabatti C, Sinsheimar J, Sobel E. Mendel version 4.0; a complete package for exact genetic analysis of discrete traits in pedigree and population data sets. Am J Hum Genet. 2001;69(Suppl):A1886. [Google Scholar]
- Lewis CM, Healey SC, Martin NG. Genetic contribution to DZ twinning. Am J Med Genet. 1996;61:237–246. doi: 10.1002/(SICI)1096-8628(19960122)61:3<237::AID-AJMG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Otsuka F, Shimasaki S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9. Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J Biol Chem. 2003;278:3713–3719. doi: 10.1074/jbc.M210598200. [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Shimasaki S. Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors bone morphogenetic protein-15 and growth and differentiation factor-9. J Biol Chem. 2004;279:17391–17396. doi: 10.1074/jbc.M401050200. [DOI] [PubMed] [Google Scholar]
- Marozzi A, Vegetti W, Manfredini E, Tibiletti MG, Testa G, Crosignani PG, Ginelli E, Meneveri R, Dalprà L. Association between idiopathic premature ovarian failure and fragile X premutation. Hum Reprod. 2000;15:197–202. doi: 10.1093/humrep/15.1.197. [DOI] [PubMed] [Google Scholar]
- Martin NG, Olsen ME, Theile H, El Beaini JL, Handelsman D, Bhattnagar AS. Pituitary-ovarian function in mothers who have had two sets of dizygotic twins. Fertil Steril. 1984;41:878–880. doi: 10.1016/s0015-0282(16)47901-x. [DOI] [PubMed] [Google Scholar]
- Martin NG, Healey SC, Pangan TS, Heath AC, Turner G. Do mothers of dizygotic twins have earlier menopause? A role for fragile X? Am J Med Genet. 1997;69:114–116. doi: 10.1002/(sici)1096-8628(19970303)69:1<114::aid-ajmg23>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mathe E, Olivier M, Kato S, Ishioka C, Hainaut P, Tavtigian SV. Computational approaches for predicting the biological effect of p53 missense mutations: a comparison of three sequence analysis based methods. Nucleic Acids Res. 2006;34:1317–1325. doi: 10.1093/nar/gkj518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Moore LG, Hudson NL, Quirke LD, Lawrence SB, Reader K, Hanrahan JP, Smith P, Groome NP, Laitinen M, et al. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction. 2004;128:379–386. doi: 10.1530/rep.1.00280. [DOI] [PubMed] [Google Scholar]
- Meulemans WJ, Lewis CM, Boomsma DI, Derom CA, Van den Berghe H, Orlebeke JF, Vlietinck RF, Derom RM. Genetic modelling of dizygotic twinning in pedigrees of spontaneous dizygotic twins. Am J Med Genet. 1996;61:258–263. doi: 10.1002/(SICI)1096-8628(19960122)61:3<258::AID-AJMG10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery GW, Zhao ZZ, Marsh AJ, Mayne R, Treloar SA, James MR, Martin NG, Boomsma DI, Duffy DL. A deletion mutation in GDF9 in sisters with spontaneous DZ twins. Twin Res. 2004;7:548–555. doi: 10.1375/1369052042663823. [DOI] [PubMed] [Google Scholar]
- Moore RK, Erickson GF, Shimasaki S. Are BMP-15 and GDF-9 primary determinants of ovulation quota in mammals? Trends Endocrinol Metab. 2004;15:356–361. doi: 10.1016/j.tem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Moron FJ, de Castro F, Royo JL, Montoro L, Mira E, Saez ME, Real LM, Gonzalez A, Manes S, Ruiz A. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS) Pharmacogenet Genomics. 2006;16:485–495. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- Nylander PP. Pituitary gonadotropins and multiple births in Nigeria. Acta Genet Med Gemellol (Roma) 1974;22:198–201. doi: 10.1017/s1120962300024707. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA. 2002;99:8060–8065. doi: 10.1073/pnas.122066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Zhao ZZ, Hoekstra C, Hayward NK, Webb PM, Whiteman DC, Martin NG, Boomsma DI, Duffy DL, Montgomery GW. Novel variants in growth differentiation factor 9 in mothers of twins. J Clin Endocrinol Metab. 2006;91:4713–4716. doi: 10.1210/jc.2006-0970. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- Vianna-Morgante AM. Twinning and premature ovarian failure in premutation fragile X carriers. Am J Med Genet. 1999;83:326. doi: 10.1002/(sici)1096-8628(19990402)83:4<326::aid-ajmg18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]