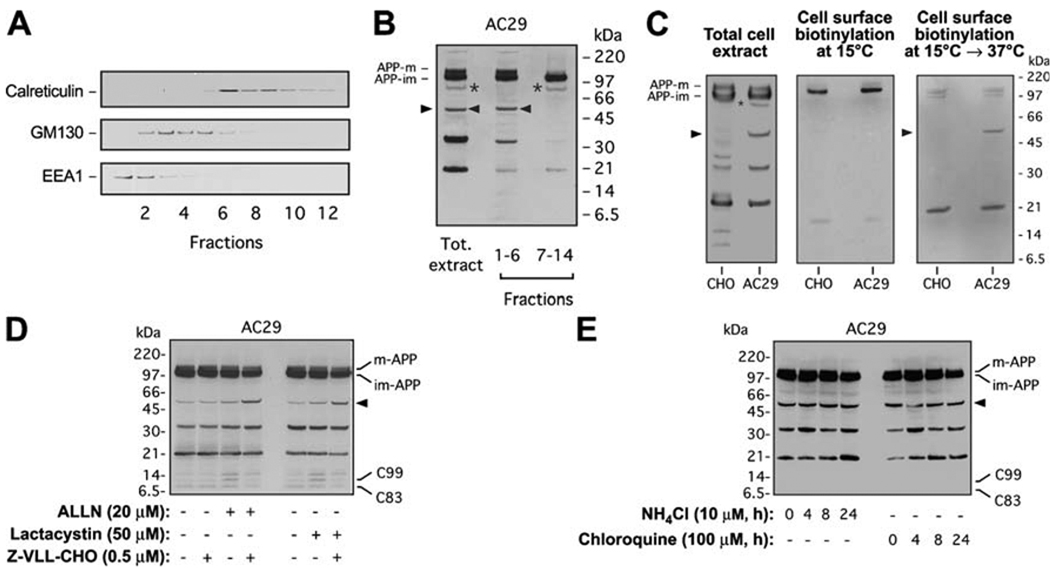
Fig. 3.
Sterol-sensitive cleavage of APP occurs in an endosomal compartment and is followed by proteasomal degradation in AC29 cells. a Cell membranes from AC29 cells stably transfected with APP were subjected to subcellular fractionation in a continuous 8–34% OptiPrep gradient. Migration of ER (calreticulin), Golgi (GM130), and early endosome (EEA1) markers in a typical gradient is shown. b Western blot analysis of APP processing was done in pooled fractions to increase the yield. 85-kDa (asterisks) and 55-kDa (arrowheads) APP-CTFs are indicated; mature (APP-m) and immature (APP-im) APP are also shown. APP-m was only detected in the fractions containing the Golgi or endosomal markers. Antibody: C7, against the C-terminus of APP. c Wild-type CHO and AC29 cells stably transfected with APP (APP expression shown in total cell extract blot on the left) were subjected to cell surface biotinylation and Western blot analysis to detect cell surface APP. Biotinylation at 15°C did not reveal generation of the 55-kDa APP-CTF at the cell surface (blot in the middle). To test if the 55-kDa APP-CTF is generated after internalization of APP holoprotein on the cell surface, cells were replaced at 37°C following biotinylation at 15°C (blot on the right). Antibody: C7, against the C-terminus of APP. d AC29 cells stably transfected with APP were treated with proteasome inhibitors ALLN (20 µM) or lactacystin (50 µM) and the β-secretase inhibitor Z-VLL-CHO (0.5 µM) for 24 h prior to Western blot analysis. Proteasome inhibition stabilized the 55-kDa APP-CTF and was able to activate the β-cleavage of the 55-kDa APP-CTF. e AC29 cells stably transfected with APP were treated with lysosomal inhibitors ammonium chloride (NH4Cl, 10 µM) or chloroquine (100 µM) for indicated times prior to Western blot analysis. Lysosomal inhibitors had no effect on the steady-state levels of the 55-kDa APP-CTF. Antibody: C7, against the C-terminus of APP