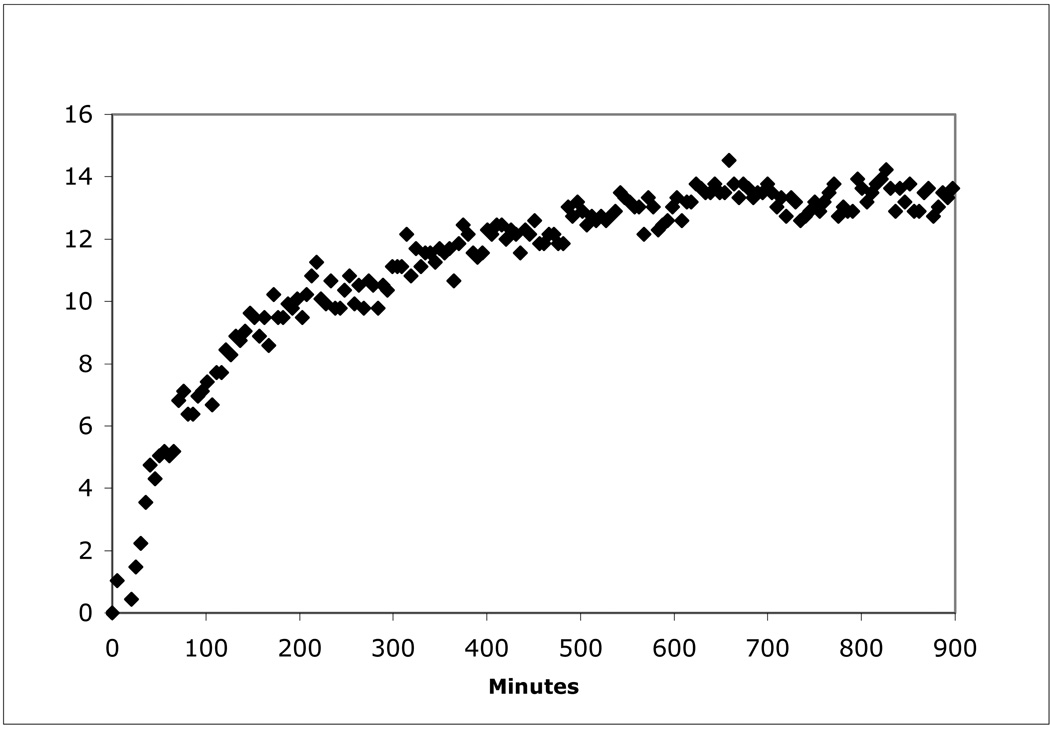
Figure 1.
Formation of Mn(III) as an intermediate in the oxidation of Mn(II) to Mn(IV) oxides as demonstrated by the pyrophosphate trapping of Mn(III) (Mn(III)-PP). Note that the concentration of Mn(III)-PP does not decrease with time suggesting it is the final product of the enzymatic oxidation of Mn(II). In the absence of Mn(II) there was no change in absorbance. The Mn(III)-PP profile shown is the result of the absorbance in the presence of 40 µM pyrophosphate minus the absorbance in the absence of pyrophosphate. Increasing the concentration of pyrophosphate leads to precipitation and increased noise in the data. The Mn(II) oxidation rate under these conditions was 2 nmol/min/mg. The lower rate can be largely accounted for by the absence of calcium in the assay.