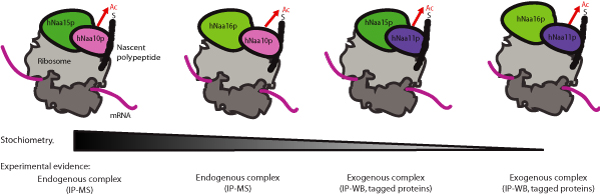
Figure 1.
Composition of the four different hNatA complexes. The hNatA subunits hNaa10p, hNaa11p, hNaa15p and hNaa16p can combine to form four variants of the hNatA complex. All subunits tested bind to ribosomes (hNaa11p not tested yet), suggesting that all four variants can acetylate nascent polypeptides (e.g polypeptide with an N-terminal Serine) co-translationally. The gradient illustrates the expected abundance of the various complexes. Based on EST data and immunoprecipitation experiments [21], hNaa10p-hNaa15p forms the most abundant version of the complex, displaying a stochiometric relationship of 6:1 compared to the hNaa10p-hNaa16p complex in HEK293 cells. The hNaa11p-hNaa15p and hNaa11p-hNaa16p complexes are probably present to an even lesser extent in most tissues, except for tissues like testis, where hNaa11p is upregulated. In the lower part of the figure it is indicated which experimental data that forms the evidence of the complex formations. IP, immunoprecipitation; MS, Mass Spectrometry; WB, Western Blotting.