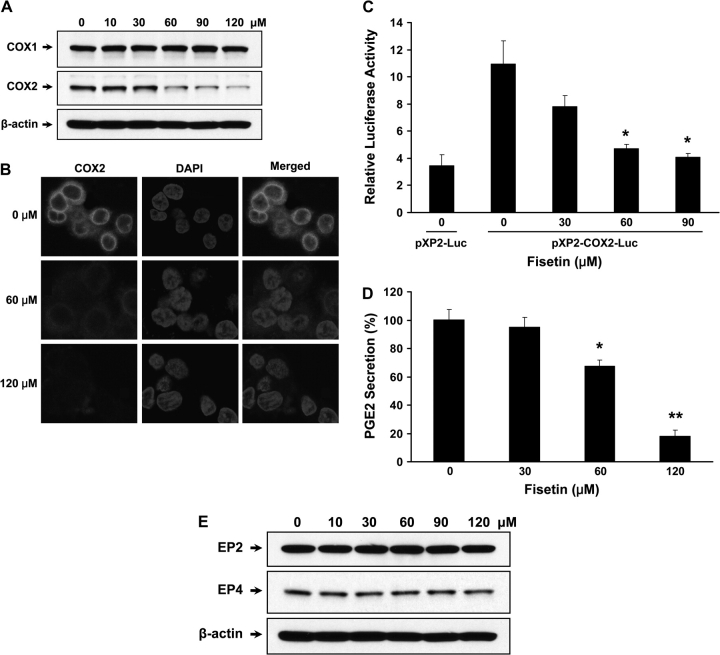
Fig. 3.
Effect of fisetin on COX expression and PGE2 secretion. Cells were treated with indicated doses of fisetin and were subjected to analysis of COX expression. (A) Western blots of COX1 and COX2 were performed with specific antibodies. The membrane was stripped and reprobed with anti-β-actin antibody to verify equal loading. (B) Immunocytochemistry using COX2-specific antibody was performed. A total of 100 000 cells were seeded and treated with indicated doses of fisetin. For immunofluorescence labeling, anti-COX2 antibody (Cayman Chemicals) and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen) were used as primary and secondary antibodies, respectively. 4′,6-Diamidino-2-phenylindole (DAPI) was used to counterstain the nucleus, ×600 (C) COX2 promoter activity with or without fisetin treatment was measured. Cells were transiently cotransfected with 1 μg of empty vector or COX2 luciferase construct containing Photinus pyralis (firefly) luciferase reporter and 50 ng of Renilla reniformis (sea pansy) luciferase control reporter plasmid. Luminescence from Renilla luciferase reporter serves as baseline response hence it was used as internal control. Results are represented as relative luciferase activity that was obtained by dividing the luminescence from firefly luciferase by the luminescence from Renilla luciferase. All samples were done in triplicate and the data are presented as mean ± SEM. *P < 0.05 versus pXP2-COX2-Luc control. (D) COX2 activity was measured by measuring PGE2 secretion. The plate was read at 414 nm using a microplate reader. The values were normalized according to cell number (1 × 106 cells) and expressed as % PGE2 secretion. The data shown are representative results of three independent experiments and are presented as mean ± SEM. *P < 0.05, **P < 0.01 versus control. (E) Western blots of EP2 and EP4 receptors were performed with specific antibodies and anti-β-actin antibody was used to verify equal loading.