Abstract
We observed the effect of modified Wendan decoction (modified Wen-Dan-Tang) on a cellular model of Alzheimer's disease. Amyloid beta (Aβ) 25–35 segment neurotoxin was employed to induce a PC12 cellular model of Alzheimer's disease. After modified Wendan decoction was fed to rats, the serum containing medicine was prepared and changes in cell morphology observed. Cell mortality and survival rate was examined by trypan blue stain assay and MTT method and caspase-3 expression was detected by western blot, while cell apoptosis was examined by flow cytometry. Cell morphology of prepared serum group was better than that of controls, and cell survival rate in prepared serum group was higher than that in control (P < 0.01 or P < 0.05). Cell mortality, caspase-3 expression and apoptosis rate in prepared serum group were lower than that in control (P < 0.01 or P < 0.05). We conclude that Modified Wendan Decoction can attenuate the neurotoxicity of Aβ 25–35 and rescue neurons via suppressing apoptotic process.
Keywords: Alzheimer's disease, apoptosis, Aβ, caspase-3, herbal medicine, PC12 cell
Introduction
Recently, neuron apoptosis has focused increasingly on exploration of Alzheimer's disease. This disease is the most common form of dementia, accounting for 50–60% of all cases (1). Deposition of senile plaque, neurofibrillary tangles inside neurons and loss of neurons in hippocampus and cerebral cortex are the prominent features of the disease. Several lines of evidence have suggested that those pathological alterations are attributed to apoptosis of neuron cells stimulated by amyloid beta (Aβ). Aβ is a key factor that forms senile plaque and an activator of microglia and astrocytes to induce neuron degeneration (1,2). Therefore, in addition to symptomatic treatment of dementia, efforts are currently used at testing the efficacy of anti-amyloid therapies (3). However, the efficacy of those efforts in clinical practice appear to be limited. Thus it is better to explore a potential way using traditional herbal medicines to treat the disease. Nowadays, PC12 cell line which is treated by Aβ 25–35 segment neurotoxin is widely used as a cellular model of Alzheimer's disease (4). This study explored the effect of Modified Wendan Decoction (modified Wen-Dan-Tang) to treat Alzheimer's disease using that cellular model.
Methods
Preparation of Modified Wendan Decoction
The related medicinal plants were collected in their respective habitat and identified by Prof. Ke-Li Chen (School of Pharmacy, Hubei College of Traditional Chinese Medicine). Then the raw plant materials were stored in the Plant Specimen Department, School of Pharmacy, Hubei College of Traditional Chinese Medicine.
Modified Wendan decoction was composed of Dangshen (Radix Codonopsis) 15 g, Zhishouwu (Radix Polygoni Multiflori Preparata) 15 g, Shichangpu (Rhizoma Acori Tatarinowii) 10 g, Tianma (Rhizoma Gastrodiae) 10 g, Chenpi (Pericarpium Citri Reticulatae) 10 g, Zhishi (Fructus Aurantii Immaturus) 10 g, Fabanxia (Rhizoma Pinelliae Preparata) 10 g, Baijiezi (Semen Sinapis Albae) 6 g, Gancao (Radix Glycyrrhizae) 4 g, Fuling (Poria Cocos) 10 g, Zhuru (Caulis Bambusae in Taeniam) 10 g, Shengjiang (Rhizoma Zingiberis Recens) 3 g and Dazao (Fructus Jujubae) 3 g. All of those herbs were decocted for 30 min after being boiled. The decoction was prepared to concentrated solution of 2.5 g ml−1 (crude drug/solution volume).
Preparation of Serum Containing Modified Wendan Decoction
Adult male Wistar rats which were 4–5 month old and 220 ± 30 g were provided from the Experimental Animal Center of Tongji Medical College (certificate No.: SYXK 2004–0028 and SCXK 2004–0007). All animals were housed under controlled conditions (22–23°C, humidity ∼70%, 12 h light, 12 h dark) for 4 days before the experiment.
Fifteen rats were collected for preparing the serum. According to the body surface area conversion and 10 times of normal dosage of humans, one rat, fasting for 12 h before administration, was intragastrically fed with the decoction of 2.5 g per 100 g per day, twice a day, for 7 consecutive days.
At 2 h after the last administration, blood was taken from heart and then serum separated by 600 g centrifugation. All the sera were mixed and filtered for sterilization and conserved at −80°C. Before using, the serum was placed at 56°C for 30 min for inactivating complement. Another 15 rats were treated with the same procedure except for the medical decoction but 0.9% sodium chloride solution. Their serum was obtained as control.
Reagents and Apparatus Preparation
Aβ 25–35 (Sigma CAS Number: 144189-71-9) was dissolved as 20 μmol l−1 in deionized water and conserved at −20°C. RPMI 1640 (Gibco) with 5% fetal bovine serum (Gibco), 10% equine serum (Gibco), 100 U ml−1 benzylpenicillin sodium, 100 U ml−1 streptomycin and 2.0 g l−1 sodium bicarbonate was prepared. Polylysine, trypsin and l-glutamic acid were purchased from Sigma. Methyl thiazolyl tetrazolium (MTT) was obtained from Fluka. RNase was provided by Sigma and anti-rat caspase-3 IgG was from Santa Cruz. CK-TKc-3 inverted phase contrast microscope (OLYMPUS), microplate photometer (Thermo Lab systems) and flow cytometer (BD FACSCalibur System) were employed for detection.
Preparation of Alzheimer's Disease Cellular Model
PC12 mouse pheochromocytoma cell line was provided from Academia Sinica. The cells were cultured with prepared RPMI 1640 medium and were set in incubator at 5% CO2, 37°C. Prior to intervention, the cells were inoculated in 96-well plate and 6-well plate with cell density of 1 × 104 and 1 × 106 When cells grew fully at the bottom of the wells, serum-free RPMI 1640 medium was used and prepared 20 μmol l−1 Aβ 25–35 were added to incubate for 24 h. At the same time, prepared serum or control serum was diluted by serum-free RPMI 1640 medium to the serum concentration of 5, 10 and 20%, and then were added to the wells. After 24 h incubation, the observation started.
Cell Morphology and Mortality Observation
Cell morphology was observed under inverted phase contrast microscope and cell mortality calculated by trypan blue assay.
Calculation of Cellular Survival Rate
MTT assay was employed to detect cellular survival rate (5), provided by the instructions of ATCC. The procedure is described briefly as: MTT was added into phosphate buffered solution (PBS) with MTT concentration of 5 mg ml−1. After the cells were incubated with Aβ 25–35 for 20 h, 10 μl MTT solution was added in each 100 μl medium and the incubation lasted for 4 h in 5% CO2, 37°C incubator. Then supernatant was drawn-off and 200 μl dimethyl sulfoxide (DMSO) was added. At last optical density (OD) value was assayed at 560 nm extinction value. The cell survival rate was calculated as: (ODmodel, prepared or control group − ODblank control)/(ODnormal medium group − ODblank control) × 100%.
Western-blot Assay on Caspase-3 Expression
Western-blot assay was employed to detect expression of caspase-3 protein expression. Treated cells were harvested and incubated on ice for 15 min in a lysis buffer of 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 100 mg ml−1 phenyl methylsulfonyl fluoride, 1 mg ml−1 aprotinin and 1% Triton X-100. Cell debris was removed by centrifugation at 10 000 rpm and 4°C for 10 min. The protein concentration of each cell lysate was determined with a Bio-Rad (Hercules, CA, USA) protein assay kit. To each tube, an equivalent volume of 2× sodium dodecyl sulfate (SDS) loading buffer (100 mM Tris–HCl, pH 6.8, 4% SDS, 20% glycerine, 10% b-mercaptoethanol and 0.2% bromophenol blue) was added and mixed again. The mixtures were then denatured at 95°C for 10 min, and about 10 mg of the protein mixture was loaded and separated in each well on 10% SDS-polyacrylamide electrophoresis gels. After separation for about 80 min, the proteins were transblotted onto nitrocellulose membranes (Bio-Rad), and the membranes saturated and blocked with 5% fat-free milk at 37°C for 1 h. Membranes were probed with rabbit anti-rat caspase-3 IgG (1 : 200 dilution) and then with horseradish peroxidase-conjugated secondary immunoglobulin G (IgG, Kangcheng, Shanghai, PR China). The membranes were then treated with an enhanced chemiluminescence reagent (Amersham, Piscataway, NJ), and the signals were detected by exposure of the membranes to X-ray films (Kodak, Rochester, NY, USA). The relative signal intensity was quantified by densitometry with Gel pro3.0 image software (Media Cybernetics, Silverspring, MD, USA) on an IBM-compatible personal computer. All experiments were independently performed three times.
Cell Apoptosis Detection
Cell apoptosis detection was performed by flow cytometry. Cells in 6-well plate with a density of 1 × 106 were collected and washed by PBS. Fixing with 70% ethanol, suspending cell with PBS, centrifuging with 600 g for 5 min was performed. Then the cells were washed and suspended again. As following, combined propidine iodide (PI) dyestuff (including PI, ribonuclease, triton X-100) was employed for one-step stain for 30 min. After being screened with 300-mesh sieve, the cell DNA was detected by flow cytometry and apoptosis rate was computed.
Statistical Analysis
One-way ANOVA analysis and independent t-test were employed to compare data. All analysis was performed by SPSS 12.0 software.
Results
Cell Morphological Observation
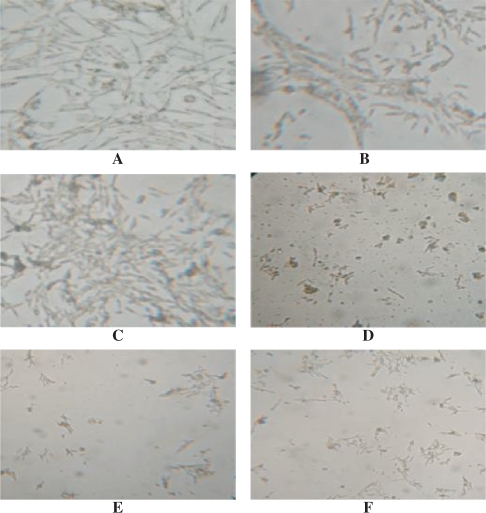
As shown in Fig. 1, when cultured without Aβ 25–35, PC12 cells in normal medium group, prepared serum group and control serum group showed fine growing, well-distributed with lucent endochylema. Cellular surrounding were fluffy with short prominence. No evident plaque and deposit could be found at the cell surface. The cell growing state in prepared serum group appeared better than that in normal medium and control serum group. In contrast, cells cultured with Aβ 25–35 showed decrease in quantity, dim and spotted surroundings. Dead, melanized and disruptive cells were observed. However, with prepared serum intervention, cell growing state was obviously better, with less dead, melanized and disruptive cells, than in normal medium and control serum groups.
Figure 1.
Morphological observation on PC12 cell (10 × 10). (A) PC12 cell cultured with normal medium. (B) PC12 cell cultured with 20% control serum. (C) PC12 cultured with 20% prepared serum. (D) PC12 cell culture with 20 μmol l−1 Aβ and normal medium. (E) PC12 cell culture with 20 μmol l−1 Aβ and 20% control serum. (F) PC12 cell culture with 20 μmol l−1 Aβ and 20% prepared serum.
Protection Against Cell Death and Promotion of Cell Survival
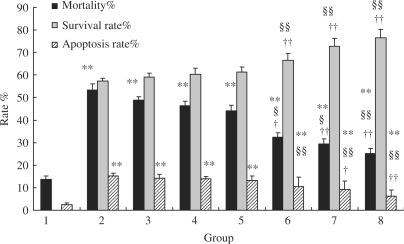
As shown in Fig. 2, we observed that compared to model and control serum groups, prepared serum lower cell mortality (P < 0.01 or P < 0.05). With the increase of prepared serum concentration, cell mortality decreased. But the mortality in prepared serum group was still higher than that in normal medium group (P < 0.01). Moreover, we observed that, compared to model and control serum group, prepared serum increased cell survival rate (P < 0.01), and more the prepared serum concentration, higher the cell survival rate.
Figure 2.
Comparison of cell mortality, cell survival rate, cell apoptosis rate (n = 8). Group 1, Normal medium; 2, Aβ model; 3, +5% control serum; 4, +10% control serum; 5, +20% control serum; 6, +5% prepared serum; 7, +10% prepared serum; 8, +20% prepared serum. The data were presented as mean ± SD. The rate was presented as percentage. Compared with normal medium group, *P < 0.05, **P < 0.01. Compared with Aβ model group, §P < 0.05, §§P < 0.01. Compared with related control serum group, †P < 0.05, ††P < 0.01.
Effect on Caspase-3 Expression
As shown in Fig. 3, cells in Aβ model group presented positive caspase-3 expression. When caspase-3 was activated, 32 kDa pro-caspase-3 was cleaved into two 17 kDa and two 12 kDa clips. In this research we detected 17 kDa clips. In prepared serum group, the caspase-3 positive expression was more notably decreasing than that in model group and control serum group (P < 0.01). But the expression of caspase-3 in prepared serum group was still higher than that in normal medium group (P < 0.01).
Figure 3.
Western-blot on aspase-3 protein expression. PC12 cell with normal medium (lane 1), PC12 cell with normal medium and 20 μmol l−1 Aβ (lane 2), PC12 cell with 20% control serum and 20 mol l−1 Aβ (lane 3) and PC12 cell with 20% prepared serum and 20 μmol l−1 Aβ (lane 4).
Comparison of Cell Apoptosis
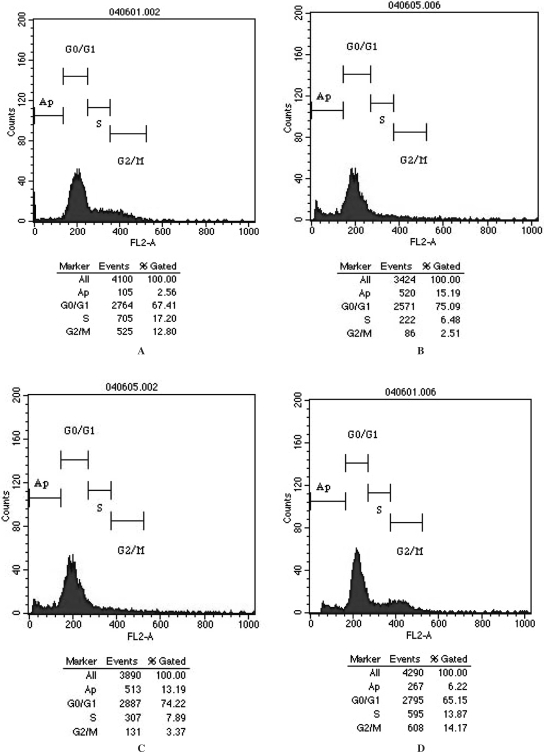
As shown in Figs 2 and 4, we observed that in Alzheimer's disease cellular model group there was a diploid caplylus at the left of G1 caplylus in flow cytometry. The apoptosis rate in prepared serum group was significantly lower than that in model group and control serum group (P < 0.01). Furthermore, the greater increase in prepared serum concentration, the greater will be the decrease in cell apoptosis (P < 0.01). However, the apoptosis rate in prepared serum group was still higher than that in normal medium group (P < 0.01).
Figure 4.
Cell apoptosis detected by flow cytometry. (A) PC12 cell with normal medium. (B) PC12 cell with normal medium and 20 μmol/l Aβ. (C) PC12 cell with 20% control serum and 20 μmol/l Aβ. (D) PC12 cell with 20% prepared serum and 20 μmol/l Aβ.
Discussion
As Aβ toxicity is regarded as the key role in pathogenesis of Alzheimer's disease, the formation of amyloid deposition in tissue is fundamental to the identification of novel therapeutic strategies to prevent or cure pathological conditions of that disorder (6). However, so far therapies towards Aβ have not been satisfactory, i.e. an Aβ vaccine clinical trial was suspended after meningoencephalitis was detected in a subset of subjects (7). There is a need to seek novel therapeutic strategies from traditional Chinese medicine to treat Alzheimer's disease.
From the 1980s, serum pharmacological research on compound herbal recipes were applied (8,9). This method adopts the serum from animals that were intragastrically administrated certain herbal medical solution. Thereby it can exclude the interference from crude medical herbs and simulate the in vivo conditions after herb administration. In this study we chose serum pharmacological method to explore the efficacy of herbal medicine to treat Alzheimer's disease.
Recently, efficacy of traditional herbal medicine to treat neurological and psychological diseases has been confirmed by many experiments and clinical trials (10–13). Wendan decoction (Wen-Dan-Tang) is a traditional Chinese compound herbal recipe recorded by ‘Bei Ji Qian jin yao fang/Essential Prescriptions Worth a Thousand Gold Coins’ which was written by Si-Miao Sun more than 1000 years ago (14,15). According to the theory of Chinese medicine, the recipe's indications include dementia symptoms which are caused by ‘turbid phlegm blocking upper orifices’. As the pathogenesis of Chinese medicine on Alzheimer's disease concerns ‘asthenia of renal essence’ as well as ‘turbid phlegm blocking upper orifices’, we added related herbal medicines in that recipe to find the formula of ‘modified Wendan decoction’.
In this exploration, P12 cell line interfered by Aβ 25–35 fragment was chosen to find a cellular model of Alzheimer's disease (4). We examined protective effect of the serum containing modified Wendan decoction with different concentration on the cellular model. The results showed that the decoction could attenuate the neurotoxicity of Aβ 25–35 and improve neuron survival via suppressing apoptotic process.
In the literature, there is no report concerning any ingredients of modified Wendan decoction to cure Alzheimer's disease. However, some efficacies of those herbs on neurological and psychological diseases have been recently revealed. Radix Codonopsis has been used as tonic agent (16,17) and Radix Polygoni Multiflori as anti-aging agent (18,19), that reduced extracellular hydroxyl radical in striatum of rats induced by intracerebral perfusion of 6-hydroxy dopamine (20). The water decoction of Rhizoma Acori Tatarinowii possessed obviously anti-depressant effect in a behavioral despair animal model of depression (21). Rhizoma Gastrodiae effectively improved the ability of learning and memory of senile rats and reduced the content of lipid peroxides (LPO) (22). Fructus Aurantii Immaturus prevented the effects of endogenous acetylcholine (23). Extract of Rhizoma Pinelliae remarkably inhibited the convulsion induced by strychnine and obviously reduced mortality rate in mice (24), but water extracts of Rhizoma Pinelliae Ternatae did not significantly decrease Aβ(1-40)-induced cell death (25). Caulis Bambusae in Taeniam was documented as anti-fatigue agent (26). The water extract of Poria cocos had the effect of bidirectional regulation on cytosolic free calcium in brain nerve cells (27). With those reports the systemically regulating efficacy of that compound herbal recipe was reflected.
Although having protective effect, the herbal medicine could not rescue all cells impaired by Aβ, which showed limits of the treatment. However, in clinical practice, as chronic progress and refractoriness is the feature for Alzheimer's disease, any fast curative effect is not expected. Therefore, the efficacy of modified Wendan decoction showed novel etiological therapeutics on this disorder and the potential pharmacological action could be explored in animal models or clinical practice.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Aisen PS. The development of anti-amyloid therapy for Alzheimer's disease: from secretase modulators to polymerisation inhibitors. CNS Drugs. 2005;19:989–96. doi: 10.2165/00023210-200519120-00002. [DOI] [PubMed] [Google Scholar]
- 3.Monaco S, Zanusso G, Mazzucco S, Rizzuto N. Cerebral amyloidoses: molecular pathways and therapeutic challenges. Curr Med Chem. 2006;13:1903–13. doi: 10.2174/092986706777585022. [DOI] [PubMed] [Google Scholar]
- 4.Nordberg A. Neuroprotection in Alzheimer's disease - new strategies for treatment. Neurotox Res. 2000;2:157–65. doi: 10.1007/BF03033791. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q, Pu P, Xia Z, You Y. Exogenous wt-p53 enhances the antitumor effect of HSV-TK/GCV on C6 glioma cells. J Neurooncol. 2007;82:239–48. doi: 10.1007/s11060-006-9279-x. [DOI] [PubMed] [Google Scholar]
- 6.Esteras-Chopo A, Pastor MT, Lopez de la Paz M. Peptide model systems for amyloid fiber formation: design strategies and validation methods. Methods Mol Biol. 2006;340:253–76. doi: 10.1385/1-59745-116-9:253. [DOI] [PubMed] [Google Scholar]
- 7.Maier M, Seabrook TJ, Lemere CA. Developing novel immunogens for an effective, safe Alzheimer's disease vaccine. Neurodegener Dis. 2005;2:267–72. doi: 10.1159/000090367. [DOI] [PubMed] [Google Scholar]
- 8.Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21:45–53. doi: 10.1016/0378-8741(87)90093-6. [DOI] [PubMed] [Google Scholar]
- 9.Umeda M, Amagaya S, Ogihara Y. Effects of certain herbal medicines on the biotransformation of arachidonic acid: a new pharmacological testing method using serum. J Ethnopharmacol. 1988;23:91–8. doi: 10.1016/0378-8741(88)90117-1. [DOI] [PubMed] [Google Scholar]
- 10.Howes MJ, Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 2003;75:513–27. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Gan AP. Clinical and psychological assessment on xinwei decoction for treating functional dyspepsia accompanied with depression and anxiety. Am J Chin Med. 2005;33:249–57. doi: 10.1142/S0192415X05002801. [DOI] [PubMed] [Google Scholar]
- 12.Weze C, Leathard HL, Grange J, Tiplady P, Stevens G. Healing by gentle touch ameliorates stress and other symptoms in people suffering with mental health disorders or psychological stress. Evid Based Complement Alternat Med. 2007;4:115–23. doi: 10.1093/ecam/nel052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott RB, Hui KK, Hays RD, Li MD, Pan T. A randomized controlled trial of Tai Chi for tension headaches. Evid Based Complement Alternat Med. 2007;4:107–13. doi: 10.1093/ecam/nel050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Y, Zhou CF. Clinical application and research of modified wendan tang decoction. Zhongguo Zhong Yao Za Zhi. 1993;18:183–5. [PubMed] [Google Scholar]
- 15.Wu J, An H, Li Y, Duan L. TCM treatment with the modified wendan tang in 40 cases of melancholia. J Tradit Chin Med. 1999;19:296–7. [PubMed] [Google Scholar]
- 16.Ngai HH, Sit WH, Wan JM. The nephroprotective effects of the herbal medicine preparation, WH30+, on the chemical-induced acute and chronic renal failure in rats. Am J Chin Med. 2005;33:491–500. doi: 10.1142/S0192415X05003089. [DOI] [PubMed] [Google Scholar]
- 17.Xu ZB, Wu J, Chen HC, Lu YJ, Gong MC. Radix codonopsis pilosulae and Radix astragali promote erythrocyte glycolysis. Chin Med J (Engl) 1987;100:654–7. [PubMed] [Google Scholar]
- 18.Xiao PG, Xing ST, Wang LW. Immunological aspects of Chinese medicinal plants as antiageing drugs. J Ethnopharmacol. 1993;38:167–75. doi: 10.1016/0378-8741(93)90012-t. [DOI] [PubMed] [Google Scholar]
- 19.Chen K, Li C. Recent advances in studies on traditional Chinese anti-aging materia medica. J Tradit Chin Med. 1993;13:223–6. [PubMed] [Google Scholar]
- 20.Wang DQ, Wang W, Zhao DZ. Effect of prepared radix polygoni multiflori on the elevation of extracellular hydroxyl radical in striatum of rats induced by intracerebral perfusion of 6-hydroxy dopamine. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:236–9. [PubMed] [Google Scholar]
- 21.Li M, Chen H. Antidepressant effect of water decoction of Rhizoma acori tatarinowii in the behavioural despair animal models of depression. Zhong Yao Cai. 2001;24:40–1. [PubMed] [Google Scholar]
- 22.Gao N, Yu S, Xu J. Improving effect of rhizoma Gastrodiae on learning and memory of senile rats. Zhongguo Zhong Yao Za Zhi. 1995;20:562–3. 568. [PubMed] [Google Scholar]
- 23.Takase H, Yamamoto K, Hirano H, Saito Y, Yamashita A. Pharmacological profile of gastric mucosal protection by marmin and nobiletin from a traditional herbal medicine, Aurantii fructus immaturus. Jpn J Pharmacol. 1994;66:139–47. doi: 10.1254/jjp.66.139. [DOI] [PubMed] [Google Scholar]
- 24.Mao S, Cheng L, Wu L. Study on anticonvulsive effect of rhizoma Pinelliae. Zhong Yao Cai. 2001;24:813–4. [PubMed] [Google Scholar]
- 25.Irie Y, Keung WM. Rhizoma acori graminei and its active principles protect PC-12 cells from the toxic effect of amyloid-beta peptide. Brain Res. 2003;963:282–9. doi: 10.1016/s0006-8993(02)04050-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Yao X, Bao B, Zhang Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam) Phytother Res. 2006;20:872–6. doi: 10.1002/ptr.1965. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, An W, Chu J. Effect of water extract of Poria on cytosolic free calcium concentration in brain nerve cells of neonatal rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1998;18:293–5. [PubMed] [Google Scholar]