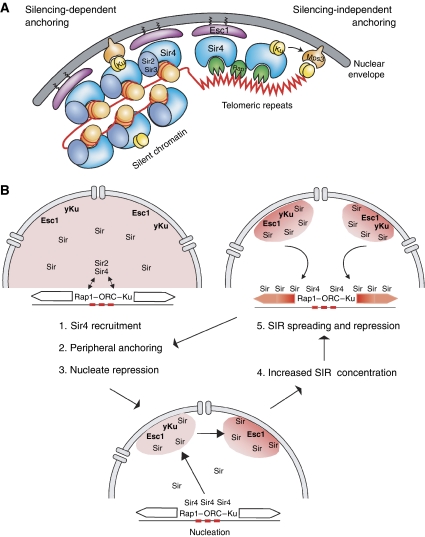
Figure 4.
Telomere anchoring and the promotion of TPE in yeast. (A) We show schematically both the silencing-dependent and the silencing- independent pathways of telomere anchoring in S. cerevisiae. The silencing-dependent pathway primarily exploits the integral SIR complex protein, Sir4, and its high-affinity interaction with Enhancer of Silent chromatin 1, which is peripherally associated with the nuclear envelope (NE). Sir4 can also bind yKu, which in turn mediates interaction with telomerase (Schober et al, 2009). Telomerase then binds Mps3, a SUN domain protein that is an integral component of the NE. At telomeres in S phase the yKu–telomerase–Mps3 pathway is sufficient to anchor telomeres in the absence of silent chromatin or Sir4 (Hediger et al, 2002). (B) We show a sequential model for how the binding of telomeres and their sequestration of SIR factors in foci can seed and the establishment of silencing at budding yeast telomeres. We propose that Sir4 is first recruited at the nucleation centre by DNA-binding proteins that can bind Sir4. These include Rap1, ORC, Abf1 and/or yKu. The presence of Sir4 at the locus will then bring it to the nuclear periphery through one of the two Sir4 anchoring pathways (yKu or Esc1) in which the high local concentrations of Sir proteins will help silencing complexes assemble and spread. Once silenced, the repressed telomere can associate with the NE through Esc1, which also increases the local concentration of Sir proteins and reinforces repression with this zone. Importantly, yKu can bind chromosome ends and link them to the nuclear envelope protein, Mps3, in the absence of repression.