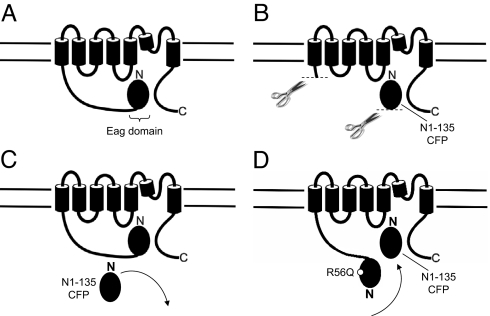
Fig. 5.
Schematic for function of eag domain. (A) Interaction of the N-terminal eag domain with other regions of the hERG channel. (B) Interaction of the eag domain with the channel is noncovalent and does not require the proximal N-terminal region. (C) Soluble eag domains do not supplant eag domain in wild-type channels. (D) Soluble eag domains supplant eag domains with point mutations, indicating that the mutation weakened an eag domain–channel interaction.