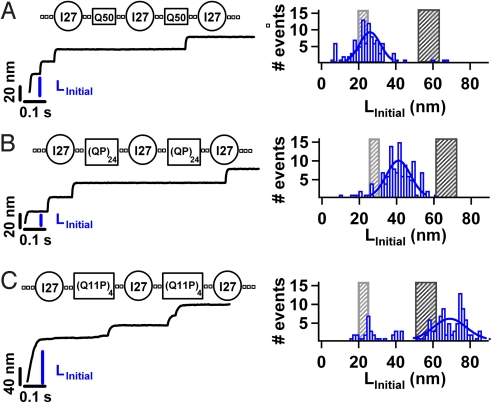
Fig. 4.
Probing the mechanical properties of heteropolypeptide chains. We construct chimeras containing the I27 protein and a heteropolypeptide chain containing glutamines interrupted by a proline residues, namely [I27-(QP)24] and [I27-(Q11P)4] (Table S1). Using force-clamp spectroscopy we apply a constant force of 180 pN along the end to end length of the constructs. We identify the full mechanical extension of a complete construct by the presence of 3 I27 unfolding steps for the (QP)24 and (Q11P)4 constructs (Table S1) where each I27 unfolding step is 24 nm in length. We measure the initial extension LInitial for each trajectory that satisfies these stringent criteria. A histogram of LInitial is shown for each of the constructs; (A) Q50 (n = 100) for comparison, (B) (QP)24 (n = 108), and (C) (Q11P)4 (n = 133). A Gaussian fit to the histograms (solid line) gives an average LInitial for each construct (QP)24 (LInitial = 42.3.1 ± 9.63 nm) and (Q11P)4 (LInitial = 70.00 ± 12.39 nm). The measured LInitial for the heteropolpeptide chain Q11P, is in agreement with the full extension of the folded I27 proteins, polypeptides, and linkers. Interestingly, LInitial for the (QP)24 construct lies between the gray and black shaded areas.