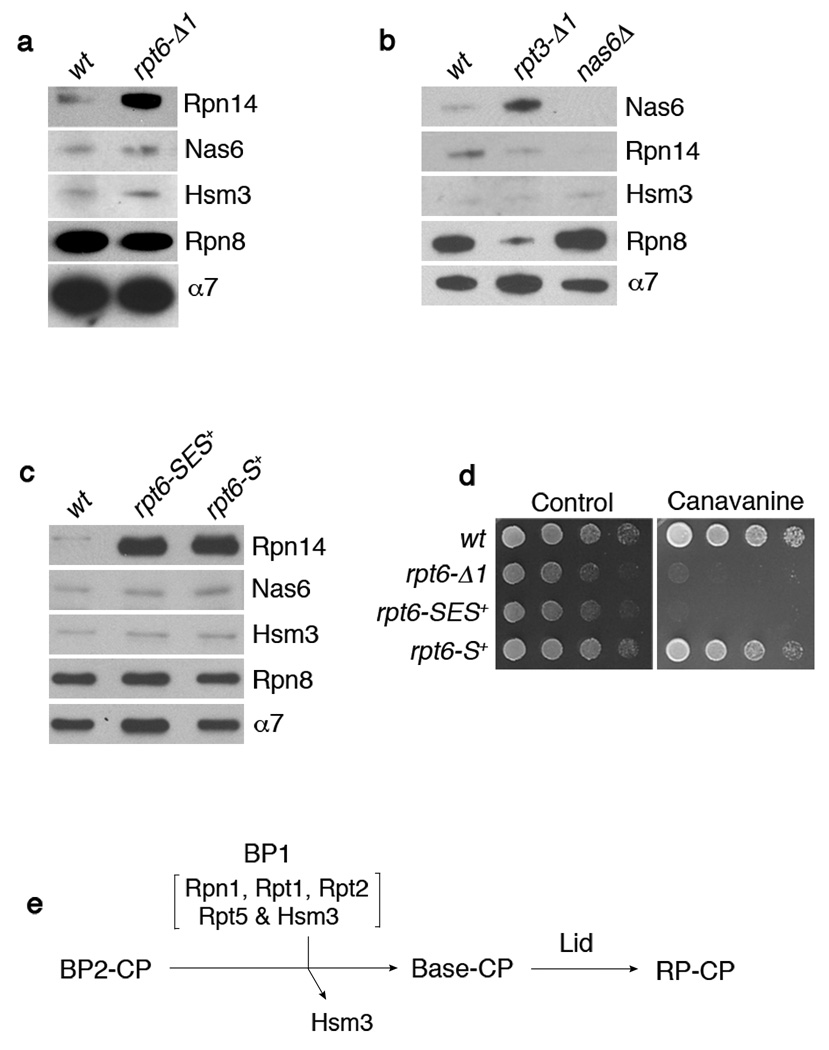
Figure 4. Interactions between Rpt C-termini and the CP control RP-chaperone release.
a-c, SDS-PAGE and immunoblotting followed by affinity purification using a CP tag (Pre1-TeV-ProA). Rpn8 (lid subunit), and α7 (CP subunit) are loading controls. Nas6Δ proteasome (b) is a negative control. The rpt6-SES+ or rpt6-S+ mutants in (c) contain either a three (Ser-Glu-Ser) or one (Ser) residue insertion prior to the fourth residue from the C-terminus.
d, Phenotypic analysis of RPT6 insertion mutants. 4-fold serial dilutions were spotted on synthetic medium (control) or canavanine (1 µg/ml) plates. Cells were grown for 2–4 days at 30°C.
e, Model for late-stage base assembly. It is proposed that the base is formed by the addition of the BP1 complex, containing Rpn1, Rpt1, Rpt2, Rpt5, and Hsm3, to the putative BP2 complex. Initial contact with the CP is provided by BP2, whereas BP1 exists independently of the CP until it joins the BP2-CP complex. BP2 has not been identified and its exact composition is unknown. However, likely components of BP2 are Rpt4, Rpt6, Rpn2, and perhaps Rpt3. Rpn14 and Nas6 (not shown) may function prior to the formation of BP2-CP.