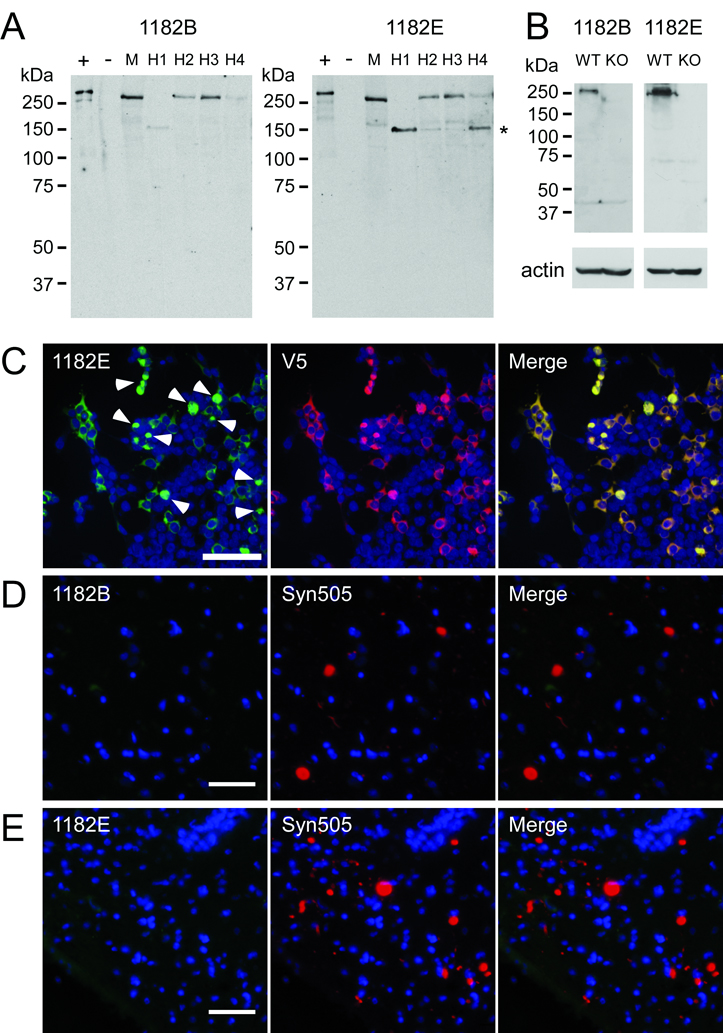
Figure 9.
Characterization of affinity-purified LRRK2 antibody. Antibody 1182 was affinity purified using peptides B and E resulting in affinity-purified antibodies termed 1182B and 1182E. (A) Western blot analysis demonstrating the ability of affinity purified antibodies 1182B and 1182E to detect endogenous LRRK2 in soluble extracts from cerebral cortex of a normal mouse (M) and 4 different humans (H1-4). Lanes + and − were loaded with 0.5 µg of soluble lysates from 293t cells transfected with LRRK2-V5 or mock cDNA (pcDNA), respectively. Lanes M and H1-4 were loaded with 100 µg of protein extracts. The mobility of molecular mass markers is indicated on the left. The major LRRK2 breakdown product is indicated with an asterisk (*). (B) Western blot analysis of wild-type (WT) and LRRK2 knockout (KO) mice with affinity purified LRRK2-antibodies, 1182B and 1182E. A specific band of the molecular weight of LRRK2 was recognized only in WT mice. Actin immunoreactivity is shown as a loading control. (C) Double immunofluorescence staining with antibody 1182E and anti-V5 antibody in 293t cells transfected with LRRK2-V5. Antibody 1182E immunolabeled both cytosolic and aggregated (arrowheads) LRRK2. (D) Double immunofluorescence with antibodies 1182B and Syn505 on cingulate cortex of a patient with diffuse Lewy body disease. (E) Double immunofluorescence with antibodies 1182E and Syn505 on substantia nigra of a patient with the LRRK2 G2019S mutation and Parkinson Disease. The affinity-purified anti-LRRK2 antibodies did not label any Lewy bodies or Lewy neurites. Bar scales: C = 100 µm; D, E = 50 µm.