Figure 1.
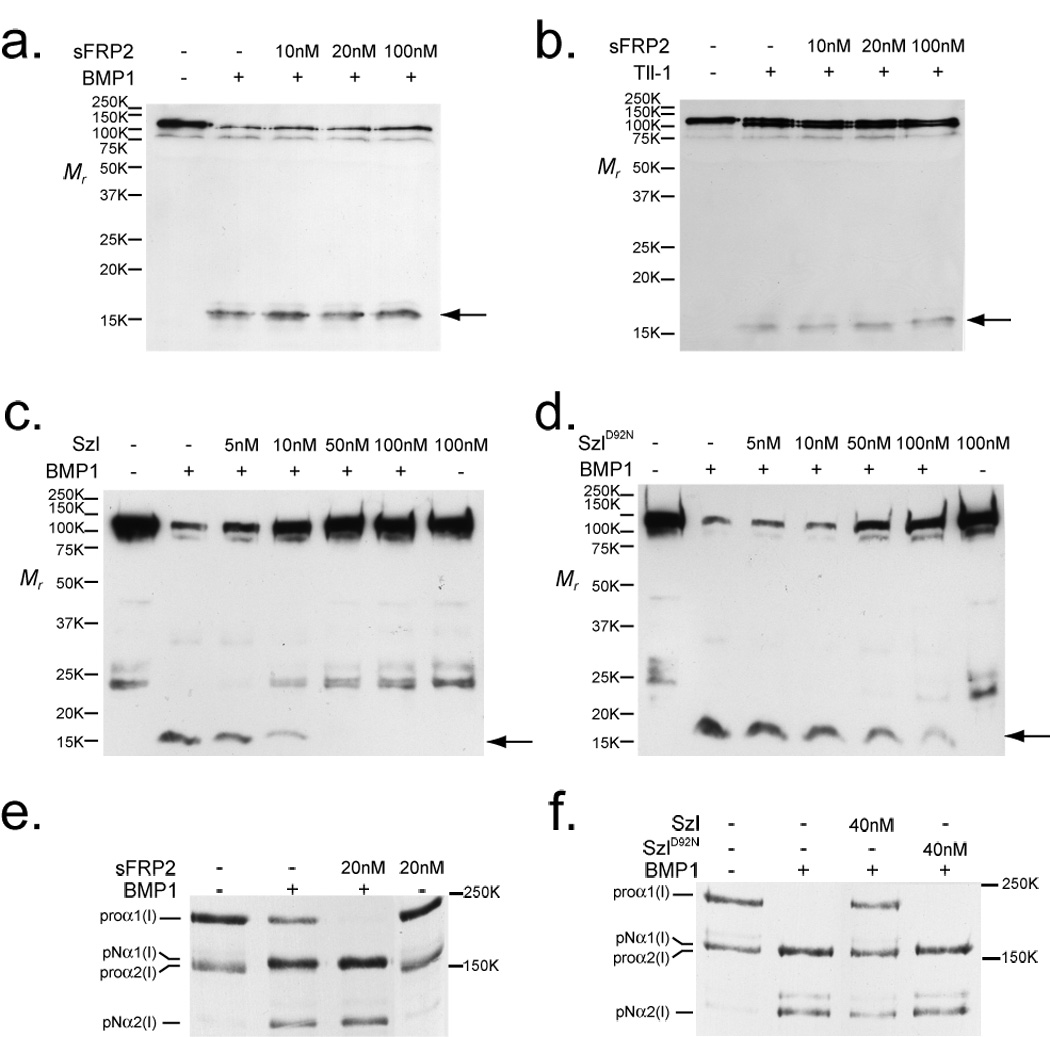
sFRP2 enhances cleavage of procollagen, but not Chordin, by BMP1 and mTLL1. Immunoblots show absence of effects of increasing concentrations of sFRP2 on the cleavage of Chordin by BMP1 (a) or mTLL1 (b), whereas Szl is shown to efficiently inhibit Chordin cleavage by BMP1 (c), with a much reduced inhibitory effect on Chordin cleavage shown by Szl with an ogon-like D92N mutation (d). Chordin cleavage is evidenced by the appearance of a 15-kDa N-terminal fragment (arrow). In contrast to the absence of sFRP2 effects on Chordin cleavage, autofluorography shows that a 2-fold molar excess of sFRP2 enhanced BMP1 pCP activity, such that no unprocessed pro-forms of the two chain classes of type I procollagen (proα1 and proα2) remained, and greater amounts of pN forms (processing intermediates retaining N-propeptides, but from which C-propeptides have been cleaved) for both chain classes were produced, compared to processing with BMP1 alone (e). Szl inhibits, and SzlD92N has no apparent effect, on BMP1 pCP activity (f). Fold-enhancement of pro-α1(I) to pNα1(I) chain processing by sFRP2 ranged from 1.2 – 2.0 fold in six independent experiments (it is 1.4-fold in fig. 1e) and in the four of these experiments in which pro-α2(I) to pNα2(I) chain ratios could be compared, fold-enhancement of pro-α2(I) processing ranged from 1.2 – 2.6 fold (such a comparison could not be made in fig. 1e, in which no uncleaved proα2(V) chains remained after cleavage of procollagen by BMP1 in the presence or absence of sFRP2). For full scans of a–f, see Supplementary Information, Fig. S6.
