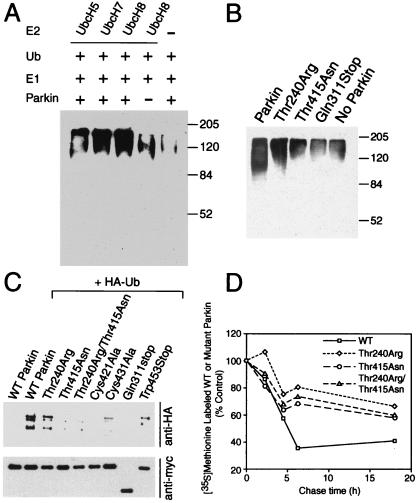
Figure 3.
Parkin is involved in E2-dependent self-ubiquitination both in vitro and in vivo. (A) In vitro translated Parkin (5 μl) was incubated with 100 ng of E1, 10 μg of ubiquitin (Ub) with 5 μl of bacterially expressed UbcH8, UbcH7, UbH5, or control bacteria lysate in the ubiquitination reaction buffer for 1.5 h at 30°C. The reaction products were immunoblotted with anti-ubiquitin antibody. Molecular weight markers are indicated. This experiment was replicated three times with similar results. (B) In vitro translated Parkin (5 μl) or Parkin mutants were incubated with 100 ng of E1, 10 μg of Ub with 5 μl of bacterially expressed UbcH8 in the ubiquitination reaction buffer for 1.5 h at 30°C. The reaction products were immunoblotted with anti-ubiquitin antibody. Molecular weight markers are indicated. This experiment was replicated three times with similar results. (C) Lysates prepared from HEK 293 cells transfected with pRK5-myc-Parkin or myc-tagged mutant Parkin constructs and pMT123-HA-ubiquitin were subjected to immunoprecipitation (IP) with anti-myc antibody followed by anti-HA immunoblotting (Upper). The blot was stripped and reprobed with the anti-myc antibody (Lower) to illustrate that equivalent levels of the Parkin constructs were present in the extracts. This experiment was replicated three times with similar results. (D) Familial-linked Parkin mutants impair Parkin degradation. HEK 293 cells were transfected with pRK5-myc-Parkin, pRK5-myc-ParkinThr240Arg, pRK5-myc-Parkin-Thr415Asn, and pRK5-myc-ParkinThr240Arg/Thr415Asn plasmids using Lipofectamine. 24 h after transfection, cells were washed and incubated with methionine-free medium for 1 h. The cells were pulsed-chased with 100 μCi of [35S]methionine and were harvested at the indicated times for immunoprecipitation with anti-myc antibody. The immunoprecipitates were resolved on a 10% SDS/PAGE gel and visualized and quantitated with a phosphoimager. This experiment was replicated with similar results.