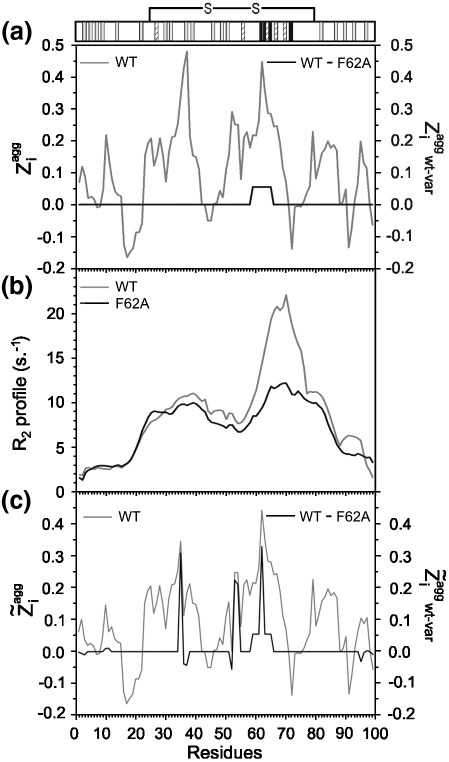
Fig. 6.
Prediction of the aggregation properties of β2m and an example variant, F62A. (a) Intrinsic propensity for aggregation of wild-type β2m (grey, left axis) as predicted by the Zyggregator algorithm modified to acidic conditions.25 The difference in aggregation propensity between wild-type β2m and its variant F62A (black, right axis). F62A is predicted by to have only a small local effect on the intrinsic aggregation propensity of β2m. A schematic illustration of the observed effects of amino acid substitution on the apparent rate of fibril elongation taken from Fig. 3d is shown above. The effects are classified as in Fig. 3, with rates not significantly altered compared with wild-type β2m (grey), 2- to 4-fold change (hashed) and > 4-fold change (black). (b) Comparison of the smoothed R2 relaxation rates for wild-type β2m and F62A β2m. Plots of R2 rates on a per-residue basis for all variants are shown in Fig. 8. (c) Comparison of the aggregation propensities of wild-type and the difference in aggregation propensities between wild-type and F62A β2m obtained by modification of the Zyggregator algorithm by the measured R2 values ().