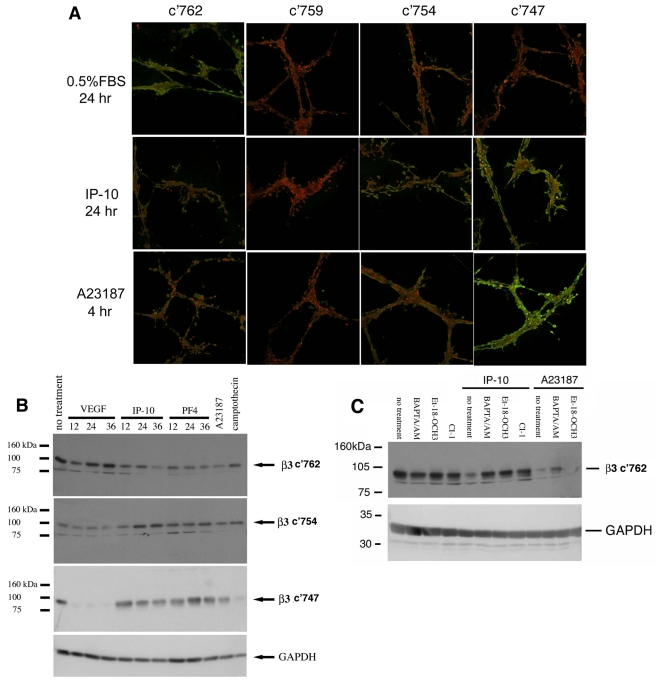
Fig. 8.
IP-10 causes cleavage of β3 integrin. (A) HMEC-1 cords were incubated with IP-10 (600 ng/ml) or A23187 (2 μM). The cords were fixed, permeabilized then stained for cleavage of β3 integrin using an antibody that recognizes β3 integrin (c'762, full length) and specific calpain cleavage sites c'754 and c'747 (Xi et al., 2003) (green) and PI (nuclear stain, red). These data indicate that IP-10 causes the cleavage of β3 integrin at the calpain cleavage site c'754 and c'747, as indicated by green staining. (B) HMEC-1 cells were grown on plastic then treated with VEGF-A (75 ng/ml), IP-10 (600 ng/ml), PF4 (400 ng/ml) or A23187 (2 μM) for various times. To analyze β3 cleavage, antibodies recognizing the C-terminal five amino acids of β3 integrin, c'762 (full length) and specific calpain cleavage sites c'754, and c'747 (Xi et al., 2003) were used. A decrease in staining of the full-length antibody (c'762) indicates cleavage of β3 integrin. Staining with the antibodies that recognize c'754 or c'747 indicates cleavage at these specific μ-calpain sites. (C) HMEC-1 grown on plastic were treated with BAPTA/AM (5 μM), calpain inhibitor 1 (CI-1, 5 mM), or Et-18-OCH3 (100 nM) prior to stimulation with IP-10 (600 ng/ml) or A23187 (2 μM). After 24 hours the cells were lysed and analyzed for β3 cleavage. A decrease in c'762 staining indicates cleavage of the cytoplasmic domain of β3 integrin. BAPTA/AM and Et-18-OCH3 are able to inhibited cleavage of β3 integrin by IP-10 (increased staining). These data indicate that cleavage is due to calpain activation. Shown is one experiment of three.