Abstract
Previously, we demonstrated that placebo analgesia (PA) accompanies reductions in neural activity during painful stimulation. This study investigated areas of the brain where neural activity increased during PA. The literature has associated PA with two potential mechanisms of action; one sustained (e.g., engaged for the duration of PA), the other, transitory (e.g., a feedback mechanism). We propose that PA results from the engagement of two complementary pain-modulation mechanisms that are identified with fMRI data as a main-effect for condition or a time*condition interaction. The mechanism with sustained activity should activate the emotional regulation circuitry needed for memory formation of the event. The mechanism with transient activity should process cognitive and evaluative information of the stimuli in the context of the placebo suggestion to confirm the expectations set by it.
To identify regions involved with these mechanisms, we re-analyzed fMRI data from two conditions, baseline (B) and PA. Results support the presence of both mechanisms, identified as two neural-networks with different temporal characteristics. Regions with sustained activity primarily involved the temporal and parahippocampal cortices. Conversely, brain regions with transient activity included linguistic centers in the left hemisphere, and frontal regions of the right hemisphere generally associated with executive functioning. Together, these mechanisms likely engage analgesic processes and then simply monitor the system for unexpected stimuli, effectively liberating resources for other process. That brain regions associated with pain modulation have different temporal profiles is consistent with the multidimensionality of PA and highlights the need for continued investigation of this construct.
Keywords: Placebo analgesia, irritable bowel syndrome, brain imaging
1 Introduction
Using fMRI, we have demonstrated that attenuation of pain related neural activity accompanies lower ratings of pain [5] during placebo analgesia (PA), consistent with our previous work [20; 21]. These results provide indirect evidence that PA results from afferent inhibition. However, these active inhibitory mechanisms of PA need further characterization, especially their temporal aspects.
An afferent inhibition mechanism blocks ascending signals from the periphery (i.e., a “gate”) [3; 10; 11]. Consistent with this well established mechanism, a number of studies have associated significant reductions in pain and pain-related neural activity during PA [17; 24]. However, simple examinations of reductions do not fully describe the mechanism by which placebos modulate pain. For example, a change in the placebo response over time suggests the presence of a mechanism with transient involvement. In three studies of PA in irritable bowel syndrome patients, the placebo response rapidly increases during the first few stimuli before reaching a plateau. A self-reinforcing feedback mechanism has been proposed to account for these temporal changes in placebo efficacy [20]. Presumably, this mechanism would compare expected versus experienced pain to confirm, maintain, and reinforce the suggested analgesic response. The first few stimuli following the placebo suggestion would be critical in the determination of how subsequent stimuli are processed. Brain regions providing this critical feedback would be more active early in the PA time course as the first few stimuli are processed. Repeated confirmation of the placebo suggestion should increase both expectations of pain reductions and PA. This hypothesis is consistent with data showing associations between increases in PA with decreases in expected pain, desire for relief, and pain-related anxiety over time [20].
We propose that PA requires input from two mechanisms, one static the other transient, to modulate pain. Cognitive and evaluative processes my be intermittently involved to confirm placebo-related expectations, and affective processes consistently encode the contextual information necessary for emotional regulation [14; 15; 17; 24; 25; 27]. Together, these two mechanisms may provide a more complete understanding of placebo analgesia and their dynamic interaction may account for individual variability in the placebo analgesia response.
To identify brain regions associated with these two mechanisms, we reanalyzed previously published baseline (B) and PA fMRI data [17]. Contrary to the previous report, which identified the brain regions where neural activity decreased as result of PA, this study investigates the brain regions where PA corresponds to an increase of neural activity, and examines activation patterns over time to identify unique temporal profiles associated with either of the two mechanisms. More specifically, we investigated brain regions with a main effect for condition (i.e., PA > B) and a time*condition interaction effect (i.e., peak activity early in the placebo condition).The results from this study provide further information about how placebos engage cognitive, affective, and sensory mechanisms to alter the experience of pain.
2 Methods and Materials
2.1 Subjects
Nine pre-menopausal women diagnosed with IBS were recruited for the study (mean age 27.7 years SD 9.6 years). Subjects included seven Caucasians, one African American, and one Hispanic. An experienced gastroenterologist using the Rome II criteria and the exclusion of organic disease [19] made the diagnosis of IBS. Six subjects had a diagnosis of diarrhea-predominant IBS, and the other three had a diagnosis of constipation-predominant IBS. At the time of the study, none of the subjects reported any clinically relevant non-IBS symptoms. Inclusion precluded the use of pain medication, SSRIs, serotonin antagonists, or tricyclic antidepressants while in the study.
The University of Florida and Gainesville Veterans Administration review boards approved this study. Prior to enrollment, all participants provided informed written consent.
2.2 Experimental materials
The gastroenterologist involved with this study was the doctor whom the majority of the patients normally consulted in the clinic. The rectal placebo agent was 200 mg saline jelly (Surgilube, E fougera and CO, Melville, NY 11747). The saline jelly was stored in traditional medical container (dispensed by the in-patient pharmacy) and applied to the rectal balloon used to induce visceral pain. The balloon used to distend the rectum was a 500 ml polyethylene bag secured to a rectal catheter (Zinetics Medical, Inc., Salt Lake City, UT) with un-waxed dental floss and parafilm, which ensured a tight seal. While the patients were in the left lateral decubitus position, the gastroenterologist coated the balloon with the saline jelly and then inserted it into the rectum so that the attached end of the bag was 4 cm from the anal sphincter. The gastroenterologist used the same lubricant in all conditions. A visceral stimulator ® (Metronics, Minn., MN) distended the rectum at a rapid rate (14.5ml/s) to a precise and constant pressure plateau between 10 and 55mm Hg, and simultaneously recorded pressure, volume, and compliance [12; 26].
2.3 Experimental paradigm
A within-subjects design was used to compare individual pain ratings between the baseline (B) and placebo analgesia (PA) conditions provided during the fMRI scanning sessions. The effect of PA was estimated with a linear contrast of pain ratings from the B and PA conditions. Patients were always tested in the B condition first, to determine individual levels of hyperalgesia (see stimulus and rating methods below) by determining which stimulus intensity that evoked pain between 40 and 60 on a 100-unit pain rating scale. Our a priori criterion for hyperalgesia was an evoked pain above 40 because 50 mm Hg distension pressures evoke much lower pain intensities in normal control subjects [20–23]. This stimulus intensity was then used in the B and PA conditions.
Sessions one and two tested pain in the B and PA conditions respectively. At the start of each session, the doctor applied the same agent (saline jelly) to the balloon just prior to insertion. During the PA condition, the doctor told the patients “The agent that you have just received is known to powerfully reduce pain in some patients.” This suggestion is identical to that used in our previous studies and is one that could be ethically applied during some active treatments [20; 21]. Patients were told in the B condition that they would receive no treatment. Because our hypothesis was that placebo responses occur during the time of stimulation, all functional brain scans included the stimulus period but not the time when pain ratings were made.
2.4 Stimulation and pain ratings
During the each neuroimaging scanning session, the rectum was distended seven times. Each rectal-distention lasted 20-seconds and was followed by a 20-second rest period. Participants rated perceived pain immediately after the 20-second distention period. Scanning sessions were spaced three to 10 days apart based on days when spontaneous abdominal pain was greater than or equal to 30. This helped to maintain consistency in pain sensitivity across sessions. See Price et al. (2007) for more information.
2.5 fMRI acquisition and analyses
2.5.1 Imaging protocol
MRI data came from a research-dedicated head scanner using a standard head RF coil (Siemens Allegra, 3.0 Tesla). High-resolution 3D anatomical images were acquired using a T1-weighted MP-RAGE protocol (128 1-mm axial slices; TR = 2000ms, TE = 4.13ms, FA = 8°, matrix = 256 × 256mm, FOV = 24cm). Functional MRI data were obtained using a T2* gradient echo planar imaging (EPI) sequence of the whole brain, which captured 33 contiguous axial slices parallel to the anterior commissure-posterior commissure (AC-PC) plane. Additional parameters were: repetition time/echo time (TR/TE = 2000 ms/30 ms), flip angle (FA) = 90°, field of view (FOV) = 240 × 240 mm, 64 × 64 matrix; 3.75 mm3 isotropic voxels with 0.4 mm slice gap. The same paradigm was repeated seven times for each experimental condition. Stimulus onset was time-locked to the onset of scan acquisition (TR). The first two volumes of each run were discarded at the scanner and two additional volumes were discarded during pre-processing to reduce saturation effects. All scanning conditions consisted of seven functional runs, each with a 20 sec non-inflation period preceding a single 20 sec stimulus. To minimize experimental confounds, scanning sessions occurred singly, on separate days, approximately a week apart.
2.5.2 Image analysis
Data were analyzed with a Xeon dual-processor 3.4GHz workstation using BrainVoyager (BVQX 1.6 - Brain Innovation, Maastricht, the Netherlands; http://www.brainvoyager.com). Image pre-processing consisted of iso-voxeling (3 mm3), rigid-body 3D motion correction using trilinear interpolation, slice-scan time correction with sinc interpolation, spatial smoothing with a 4-mm full-width at half maximum (FWHM) Gaussian kernel, voxel-wise linear detrending, and high-pass temporal filtering to remove nonlinear drifts below 3 Hz. The functional images were co-registered to a high resolution 3D anatomic volume and transformed into standard Talairach space [18]. During spatial transformation, functional voxels were interpolated to a resolution of 1 mm.
2.6 Analytic strategy
A multi-step analytic approach was used to identify brain regions associated with the sustained and transient mechanisms of placebo analgesia.
A Random Effects (RFX) General Linear Model (GLM) was used to identify cortical regions wherein pain-stimulus onset was significantly convolved with the hemodynamic response function (HRF). Aside from the main effect of the balloon inflation, several specific contrasts were used to compare the magnitude of BOLD response across the Baseline and Placebo Analgesia conditions. As a precaution against Type I error, resultant statistical parameter maps (SPMs) were thresholded at p <.005, and had a spatial-extent of 50 contiguous voxels (i.e., 50-µL in volume). These criteria result in a probability of detecting a false positive pixel, per pixel, at 0.000001 [7]. The SPMs were then overlaid on a standardized 3D anatomical volume for localization. Specific hypotheses and additional information regarding our analytical approach is outlined below.
2.6.1 Hypothesis-1: Placebo > Baseline (sustained placebo involvement)
A more thorough evaluation of condition-related effects was done by examining changes in BOLD within specific volumes of interest (VOIs). To increase construct specificity and contrast sensitivity of the VOI analyses, the geographical extents of the VOIs were based on the t-contrast of the Baseline and the Placebo Analgesic condition. Active voxel clusters were classified as a specific VOI under the following conditions: voxels survived second-level threshold of p <.005, maintained a spatial-extent of 50 contiguous voxels, and the center-of-gravity of the cluster could be identified (e.g., not in CSF). Once the geographical extents of each VOI were determined, only those voxels within the VOI were used in the subsequent condition-contrast analyses. Thus, each VOI provided a small volume correction (SVC) and reduced the overall number of voxel-wise comparisons. The VOIs also increase sensitivity in detecting BOLD differences between conditions via VOI-specific RFX-GLMs.
To assess condition-based effects (e.g., placebo analgesia) the ROI-RFX-GLM used all the voxels in the VOI rather than just those that were significant for the within-condition contrast; this analysis hedged against the possibility that no voxels survive the within-condition contrast (i.e., inflated vs. not inflated). Brain regions identified by this analysis should be linked to a mechanism with a sustained involvement in the placebo analgesic response.
2.6.2 Hypothesis-2: Early > Late Placebo activity (transient placebo involvement)
Although the VOIs identified by hypothesis #1 would be consistently more active during the placebo condition, the magnitude of that involvement may systematically change over time. Consequently, within the placebo condition, for each of the VOIs, we compared the brain activity during the first three rectal distention periods (early) to the last three rectal distention periods (late). The fourth distention was included as a predictor of no interest. The within condition threshold for statistical significance can be changed to p < .05 because the criteria used to identify the VOIs in the initial contrast was so conservative (see above).
2.6.3 Hypothesis-3: Time by condition interaction (condition specific transient involvement)
Brain regions uniquely associated with a self-reinforcing placebo mechanism should have transient properties specific to the placebo condition. We used a Two-Factors Repeated Measures ANOVA to test this assumption twice. Initially, we used just the data from the VOIs identified in Step-1 to test for time*condition interaction effects. We followed up those tests with a whole-brain analysis to identify brain regions with the specified time*condition temporal profile. This second analysis was done to identify brain regions not typically associated with pain, but may nonetheless contribute to the self-reinforcing placebo mechanism.
3 Results
3.1 Psychophysical - pain ratings
As previously reported, the pain ratings from all seven balloon inflations were used as dependent measures in a repeated measures ANOVA with condition (B and PA) as one within subject factor, and balloon trial as a second within-subject factor [17].
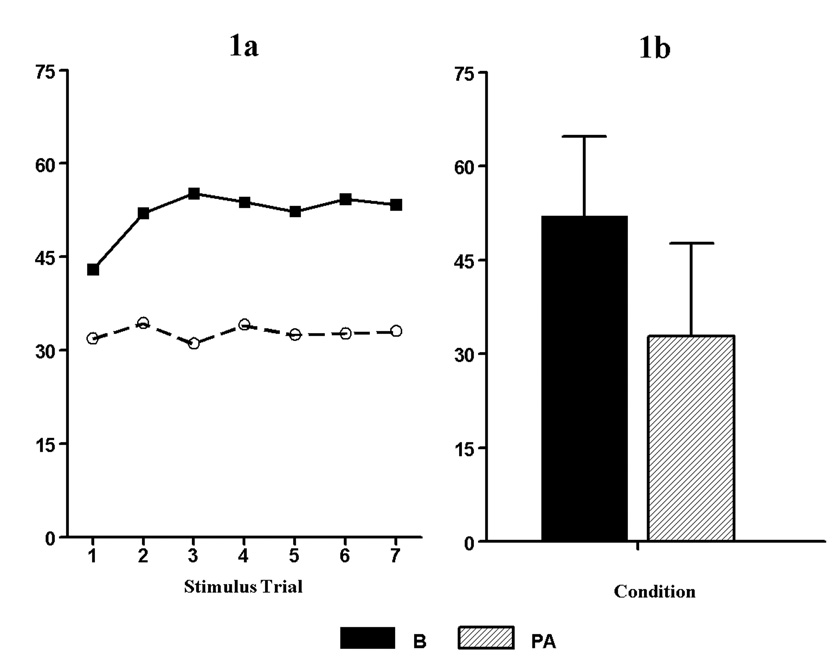
Results of the repeated measures ANOVA for pain ratings revealed a significant main effect for condition (F (1, 8) = 51.5, p < .000, η2 = .87). There was also a main effect for stimulus trial (F (6, 48) = 2.45, p < .038, η2 = .23). However, there was no condition by trial interaction (F (6, 48) = 1.25, p > .05). These results demonstrate our success in achieving a placebo effect.
3.2 Neuroimaging - placebo-specific neural activity
The results for the three hypotheses are presented in order below.
3.2.1 Hypothesis-1: Placebo > Baseline (sustained placebo involvement)
The whole-brain RFX-GLM i) identified regions in the brain where neural activity was significantly associated with rectal distension from the Baseline and Placebo Analgesic conditions (i.e., inflated > not inflated); and ii) identified volumes of interest (VOIs) exhibiting a significant main effect for condition (i.e., PA > B). The results from this contrast imply that the VOIs identified were significantly involved in the analgesic response in a sustained manner. A number of VOIs were identified in both hemispheres and included frontal (cognitive) and limbic (emotional) structures as well somatosensory and association areas of the cortex. See Table 1.
Table 1.
Placebo Responsive Regions (PA > B)
| Region | X | Y | Z | µL |
|---|---|---|---|---|
| Right Pre-Central Gyrus | 44 | −13 | 28 | 527 |
| Right Superior Temporal Gyrus | 35 | −52 | 12 | 103 |
| Right Parahippocampal Gyrus | 23 | −35 | −18 | 77 |
| Right Amygdala/Parahippocampal Gyrus | 18 | −10 | −17 | 182 |
| Right Posterior Cingulate | 7 | −68 | 17 | 86 |
| Left Pre- Cuneus | −26 | −70 | 18 | 207 |
| Left Superior Temporal Gyrus | −36 | −55 | 13 | 54 |
| Left Middle Frontal Gyrus | −40 | 9 | 32 | 154 |
| Left Middle Temporal Gyrus | −42 | −62 | 17 | 84 |
| Left Post Central Gyrus | −48 | −20 | 40 | 70 |
3.2.2 Hypothesis-2: Early > Late Placebo activity (transient placebo involvement)
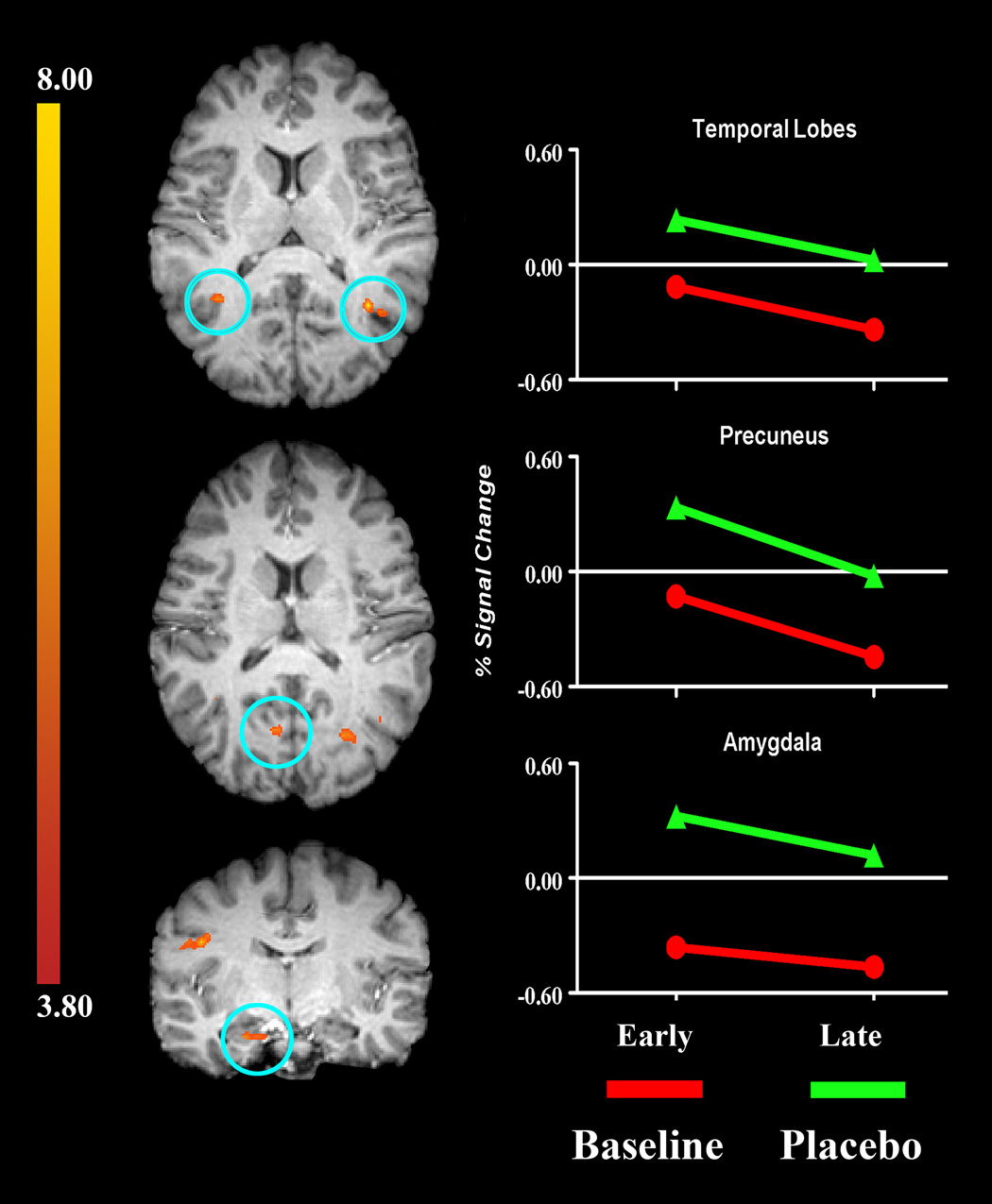
Because the analgesic efficacy of placebo may increase over time, within the placebo condition, we analyzed the time-course of the VOIs a main effect of time. Consistent with the theory of a self-reinforcing feedback mechanism, 8 of the 10 VOIs with sustained placebo involvement, were significantly more active during the first three stimuli (p < .05; i.e., a main effect for condition and time) See Figure 2. Two VOIs did not exhibit a main effect for time. The BOLD signal in the right parahippocampal gyrus and the left middle frontal gyrus was consistently greater in the B vs. PA condition (i.e., main effect for condition only). See Table 2.
Figure 2.
Brain regions identified as having i) a main effect for condition (Placebo >Baseline), and ii) a main effect for time (first three stimuli [Early] > [Late] last three stimuli). Brain regions circled in blue are (from top to bottom): bilateral temporal lobes, left precuneus, and the right amygdala.
Table 1.
Step-1 VOIs statistics (Main effect for Time and the Time*Condition interaction)
| Region | Early > Late1 | Early > Late2 |
|---|---|---|
| Right Pre-Central Gyrus | 0.008 | 0.739 |
| Right Superior Temporal Gyrus | 0.010 | 0.923 |
| Right Parahippocampal Gyrus | 0.326 | 0.301 |
| Right Amygdala/Parahippocampal Gyrus | 0.004 | 0.489 |
| Right Posterior Cingulate | 0.038 | 0.590 |
| Left Pre- Cuneus | 0.000 | 0.777 |
| Left Superior Temporal Gyrus | 0.008 | 0.934 |
| Left Middle Frontal Gyrus | 0.199 | 0.731 |
| Left Middle Temporal Gyrus | 0.000 | 0.822 |
| Left Post Central Gyrus | 0.030 | 0.263 |
p-value for the main effect of time within the placebo condition
p-value for the time by condition interaction
3.2.3 Hypothesis-3: Time by condition interaction (estimating unique temporal profiles of PA pain-modulation)
Research suggests that placebo suggestions for pain reduction, expectations, and positive affect, influence the experience of pain [16; 20; 21], which are consistent with the results of hypothesis #1. Additionally, results of the second hypothesis suggest that placebo analgesia may result from the early influence of (positive) affect on processing pain-related information. However, it was unknown whether this early affective involvement was common to processing painful stimuli or unique to the placebo condition. Consequently, we tested for a time*condition interaction among the VOIs identified by hypothesis #1. Despite a main effect for condition, and contrary to our hypotheses, the results of hypothesis #3 failed to identify a significant time (early vs. late) by condition (PA vs. B) interaction effect among the 10 VOIs (Table 2). The lack of an interaction effect among these VOIs suggests that additional brain regions are involved in pain-modulation and placebo analgesia.
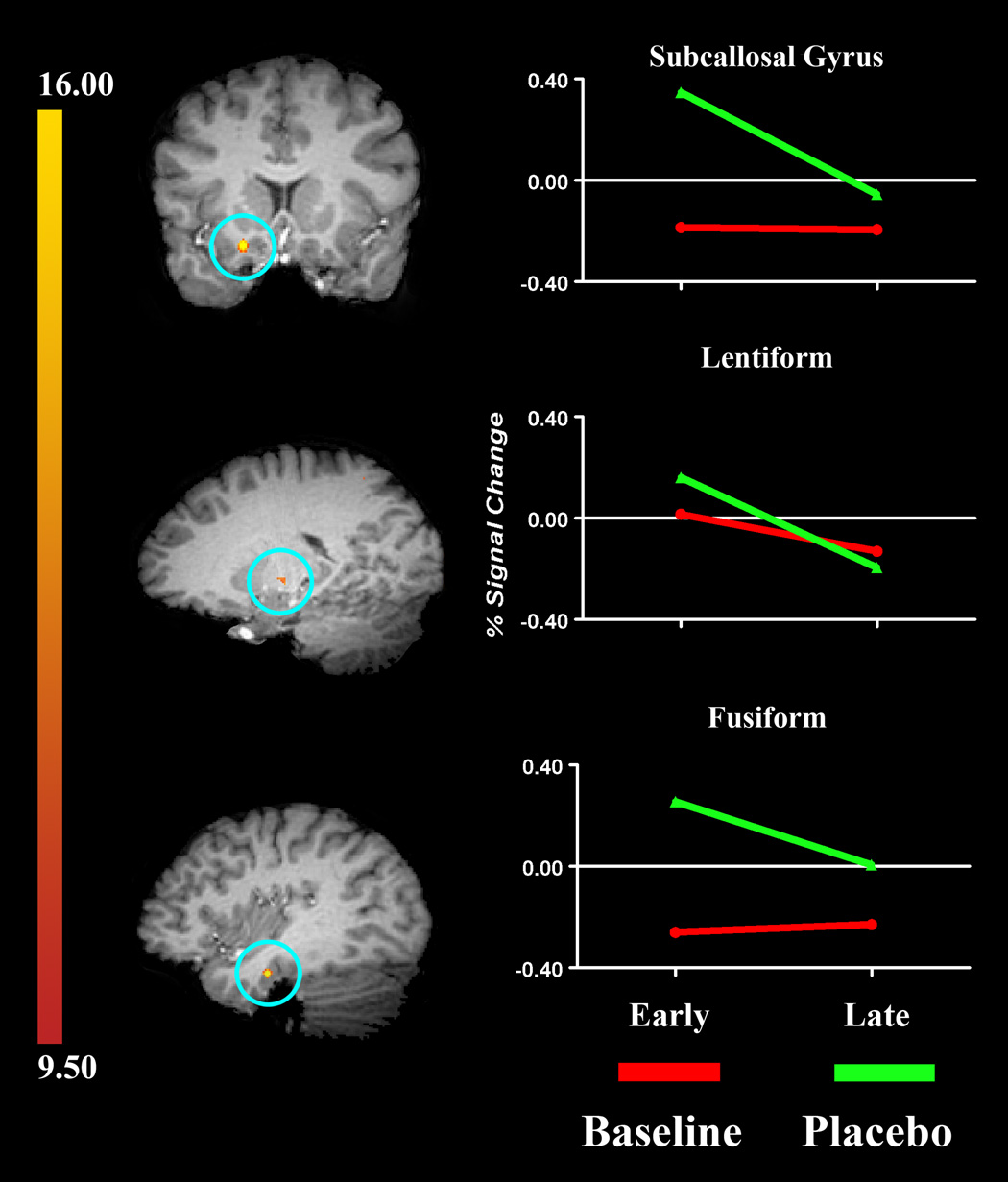
To identify these additional sources of pain-modulation, we re-analyzed the whole brain to identify brain regions with a significant early-placebo time*condition interaction profile. This analysis identified eight brain regions that met our statistical criteria (Step-3 VOIs). All eight of the clusters identified had probabilities less than 0.000001 for detecting a false positive. The brain regions with neural activity matching this profile included aspects of the frontal lobe (precentral gyrus), cingulate, insula, and temporal lobe (fusiform gyrus), see Table 3 and Figure 3. These eight VOIs are considered uniquely involved in the early phase of the PA feedback-mechanism, likely for the establishment of placebo analgesia. Because the neural activity peaked early in the PA time course, it is possible that these brain regions involved with the mechanism that transforms the placebo suggestion into an endogenous analgesic.
Table 1.
Regions with an early PA time*condition interaction (whole brain analyses)
| Region | X | Y | Z | µL | F | p-value |
|---|---|---|---|---|---|---|
| Right Frontal Lobe Precentral Gyrus | 63 | −5 | 18 | 52 | 24.314 | 0.001 |
| Right Frontal Lobe Precentral Gyrus | 33 | −10 | 25 | 44 | 21.753 | 0.002 |
| Right Frontal Lobe Subcallosal Gyrus | 18 | 14 | −12 | 63 | 37.578 | 0.000 |
| Left Cerebellum | −2 | −46 | −16 | 40 | 18.528 | 0.003 |
| Left Limbic Lobe Cingulate Gyrus | −8 | −17 | 26 | 126 | 28.993 | 0.001 |
| Left Lentiform Nucleus | −17 | −13 | 0 | 35 | 26.492 | 0.001 |
| Left Temporal Lobe Fusiform Gyrus | −33 | −40 | −14 | 30 | 30.886 | 0.001 |
| Left Post Central Gyrus | −36 | −25 | 23 | 26 | 15.801 | 0.004 |
Figure 3.
Brain regions identified as having a significant time*condition interaction (i.e., Early [PA] > all other cells). Brain regions circled in blue are (from top to bottom): right subcallosal gyrus, left lentiform nucleus, and left temporal lobe. The graphs on the right show the amount of signal change across time (early to late). Note: In both conditions, the Late signal intensity values reflect the amount of change that has occurred from the Early time-period, which have been rescaled to a value of zero.
3.3 Additional analyses
Neuroimaging studies of placebo analgesia frequently report the involvement of the PAG, rostral anterior cingulate cortex, and left amygdala. Consequently, we expected these regions to be highly active during the PA condition. Yet, there was no main effect of time, condition, or a time*condition interaction for these regions at the p <.005 level. However, using a less conservative criterion (i.e., p < .05; probability of a false positive = .00002), we found that these regions did contribute to the placebo response. While these regions contributed to the PA response, their reduced statistical significance in comparison to the main results highlights relative contribution of the VOIs to the placebo response.
At the p<.05 level, we found support for a main effect for condition (PA), but not for time, or a time*condition interaction in the rostral anterior cingulate cortex (p < .39 and .25 respectively). We also identified a main effect for condition (PA) and time (early), but found no support for a time*condition interaction (p<.09) in the left amygdala. Despite being less intense, the pattern of neural activity in the left amygdala appeared to mirror that of the right amygdala, suggesting a similar role for both amygdalae. The neuronal activity of the PAG was statistically equivalent in both the B and PA conditions. The magnitude of activity may not differ across conditions if they are involved in different processes (e.g. afferent processing in B and descending modulation in PA).
4 Discussion
The brain regions that demonstrated an increase in neural activity, coinciding with placebo analgesia, including those identified in the less conservative “additional analyses” are associated with at least two general mechanisms of pain-modulation. The first appears to engage affective processes during the entire placebo condition to aid in pain-modulation and includes such regions as the rostral ACC, bilateral amygdala, and medial prefrontal cortex. The second may engage higher order/cognitive processes early in the placebo time course for the purposes of context evaluation, providing feedback about expectation-stimulus correspondence, and various tertiary processes, such as associative thinking. Such regions include the posterior cingulate, pre-cuneus, rostral ACC, perihippocampal gyrus, and aspects of the temporal lobes. The remaining discussion will focus on those effects and areas specifically related to main effects of condition, temporal development of the placebo effect, and their interaction.
4.1 Placebo analgesia is accompanied by sustained increases in brain activity
Compared to the baseline (B) condition, placebo analgesia (PA) accompanied a sustained increase in neural activity among pain modulatory areas bilaterally. Brain regions with sustained involvement in PA included medial prefrontal, posterior cingulate, bilateral aspects of the temporal lobes, amygdala, and perihippocampal cortices. The sustained engagement of these regions during PA likely represents their involvement in: i) afferent inhibition via pain-inhibitory mechanisms that descend from the brain to the spinal cord [3; 6; 10; 17], and ii) the decrease in activation among pain-related brain regions during placebo analgesia [17; 24]. Many, but not all of these brain regions have been associated with PA in previous works [8; 24] (see Table 1). Elevated activity in these newly identified pain-modulatory regions may well reflect the engagement of higher-order (psychological) processes that are needed to link affective and contextual information during the formation of episodic memories [9]
4.2 Placebo analgesia is accompanied by transient increases in brain activity
We proposed the presence of another mechanism, one with transient temporal properties that would complement the first mechanism in the modulation of pain. This would be a self-reinforcing feedback mechanism [20]with a specific temporal profile of neural activation. Brain regions associated with this mechanism may or may not be consistently engaged during the experience of placebo analgesia. Among the 10 brain regions with significant placebo related activation, there was significantly greater activity earlier in the temporal profile for eight of the 10 placebo-related VOIs (see Table 2). Brain regions actively involved with the early aspects of the PA response included: bilateral aspects of the insula, cingulate, temporal lobes, and Lentiform nuclei. These regions are known to be involved with different aspects of limbic/affective, somatosensory/sensation, and frontal/cognitive information processing.
These results implicate the early involvement of multiple information processing networks and highlight the complexity of endogenous pain modulation. However, among the VOIs in Table-2, the early peak in activity was not unique to the placebo condition. The lack of a condition effect suggests that these brain regions are involved in the modulation of pain in both PA and B conditions.
We used an exploratory analysis of the whole brain to identify brain regions involved in pain modulation, but were not consistently engaged during placebo analgesia. Specifically, we sought to identify regions with a significant early-placebo time*condition interaction. This analysis yielded eight VOIs that matched this temporal profile and included aspects of the cingulate, parietal, and temporal cortices, the latter of which is commonly associated with neurolinguistic processes (Table 3 and Figure 3). The brain regions identified by this analysis are noteworthy for two reasons. First, the majority of the VOIs engaged early in the experience of placebo analgesia are typically associated with cognitive and emotional processes. Second, there was more involvement of the left compared to the right hemisphere in the early aspects of placebo analgesia suggesting that some aspects of the placebo response are verbally mediated.
4.3 Placebo analgesia is multi-mechanistic
Studies of placebo analgesia (PA) have associated the analgesic response to neural networks involved in cognitive and affective information processing [8; 9]. In the present study, we show that placebo analgesia accompanies sustained increases in neural activity during painful stimulation for the duration of the PA time course. These results are consistent with the idea that PA is the result of sustained inhibition of afferent input to thalamic and cortical structures (.e.g. descending inhibition to spinal cord). We also found support for a placebo analgesic mechanism that involves a self-reinforcing feedback loop. This feedback mechanism would exert its greatest influence during the first few stimuli, when an assessment about the correspondence between expectation and sensation occurs. Congruence would reinforce the expectation, increase the effectiveness of cognitive/affective factors to mediate the experience of pain, and enhance the efficacy of placebo response.
Support for both placebo-related mechanisms suggests that they are complementary processes, working together in the production and maintenance of (endogenous) placebo-analgesia. Further, the identification of multiple brain areas with variable temporal involvement in the modulation of pain is consistent with reports that placebo analgesia is multi-modal, and requires the integration and synthesis of multiple sources of information [8; 24].
Several brain regions previously implicated in “associative thinking” and considered part of a “default” network were more active in the PA condition than in the B condition. These included right posterior cingulate, left precuneus/ post-cingulate, left middle frontal gyrus and parts of the posterior temporal lobe (temporoparietal junction). This network of regions becomes activated during associative thinking and deactivated during specific tasks requiring directed attention [2]. Associative thinking has been described as a conscious background state wherein subjects make unconstrained associations that are unrelated to the immediate external environment [2]. If so, these brain areas are more active during the PA condition than during the B condition and are more active during the early part of both B and PA conditions. This pattern may help explain the dynamics of placebo analgesia. During the PA condition, participants were likely to be making associations between prior suggestions about the placebo agent, internal cues that suggest whether or not the agent is working, and their expectations about future pain experience. These associations require memory, somatic focus, and comparison of present experience to expectation of pain following placebo suggestion. This conceptualization is consistent with a self-enhancing feedback mechanism [21]. A similar dynamic also would naturally occur largely during the early periods of both B and PA conditions. The early period is likely to reflect a time when the search for cues (somatic focusing) that predict future experience and their associations with past events (e.g. instructions, suggestions) are likely to be the most salient. This explanation would apply to both B and PA conditions.
4.4 Implications and future studies
The concept of placebo has shifted in emphasis from the idea that placebo effects are the result of inert agents to the idea that placebo effects are sensitive to the influence of psychological constructs such as the expectations, beliefs, and desires of patients. Placebo analgesic effects have been shown to vary in magnitude through the manipulation of these variables [1; 4; 13; 16]. In addition to clarifying the dynamic relationships among the brain regions associated with the placebo analgesic mechanisms, future research will need to help delineate the limits of PA. The extents to which placebo analgesia is sustainable via subtle changes in the placebo suggestion, whether these changes correspond to meaningful changes in pain ratings, and are detectable in fMRI data remains in question.
Figure 1.
A) Mean pain ratings for the 7, 20-second rectal distensions produced by a balloon barostat in the baseline (B) and placebo analgesic (PA) conditions. Note: the pain ratings in the B condition increased over time whereas the pain ratings in the PA condition were relatively stable.B) Mean pain ratings (standard deviation) of the last five stimuli were (B= 52.0 (12.7) and PA= 32.8 (14.9).
Acknowledgments
Support for this research was provided from Grant RO1 (AT001424) to Dr. Michael Robinson, from the National Institutes of Health, National Center for Complementary and Alternative Medicine, a Merit Review Award (PI: GN Verne) from the Medical Research Service of the Department of Veteran Affairs, and Grant 1-R01-NS053090-01 from the National Institutes of Health (PI: GN Verne). The authors would also like to thank Adam Hirsh, Erin O’Brien, Karen Chung, Xeve Silver and Trish Stamm for their assistance in data collection. There is no relationship (financial or otherwise) that poses a conflict of interest, and thus precludes the publication of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neuroscience. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar M, Aminoff E, Mason M, Fenske M. The units of thought. Hippocampus. 2007;17:420–428. doi: 10.1002/hipo.20287. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson ME. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields HL, Price DD. The placebo effect: An interdisciplinary exploration. In: Harrington A, editor. Toward a neurobiology of placebo analgesia. Cambridge, MA: Harvard University Press; 1997. pp. 93–116. [Google Scholar]
- 7.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 8.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer DJ, Price DD. Central nervous system mechanisms of analgesia. Pain. 1976;2:379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- 11.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 12.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 14.Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- 16.Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Prog Brain Res. 2000;122:255–271. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- 17.Price DD, Craggs JG, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-Planar stereotaxic atlas of the human brain. New York: Thieme Publishers Inc; 1988. [Google Scholar]
- 19.Thompson WG. Irritable bowel syndrome: a management strategy. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:453–460. doi: 10.1053/bega.1999.0039. [DOI] [PubMed] [Google Scholar]
- 20.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 22.Verne GN, Davis RH, Robinson ME, Gordon JM, Eaker EY, Sninksy CA. Treatment of chronic constipation with colchicine: randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2003;98:1112–1116. doi: 10.1111/j.1572-0241.2003.07417.x. [DOI] [PubMed] [Google Scholar]
- 23.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 24.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 25.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human {micro}-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehead WE, Cheskin LJ, Heller BR, Robinson JC, Crowell MD, Benjamin C, Schuster MM. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98:1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]
- 27.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]