Figure 4. LiRP Proteins Inhibit AMPK and Reduce the AMP Stimulation of the Kinase.
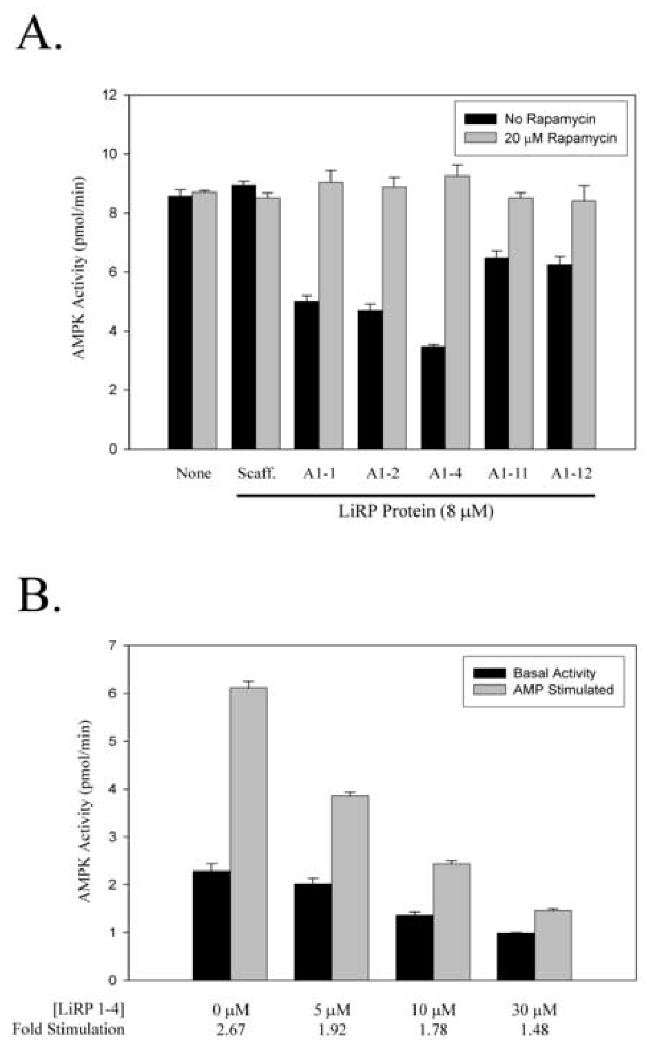
A. Graph showing the in vitro kinase activity of AMPK under varied conditions. Dark bars indicate the kinase activity in the absence of Rapamycin while light bars are the activity in the presence of 20 μM Rapamycin. Kinase activities were determined in the absence or presence of various LiRP proteins (A1-1, A1-2, A1-4, A1-11, A1-12), or the LiRP scaffold protein with a sequence not selected for binding to AMPK α1 (Scaff insert sequence is LYCYE). All LiRP protein concentrations are 8 μM, and 10 mU of purified AMPK (Upstate Biotech) was used (10 mU at 759U/mg protein in 25 μL reactions equals less than 3 nM AMPK heterotrimeric complex). B. AMPK kinase assays depicting the LiRP A1-4 inhibition of AMPK activity in the presence or absence of 200 μM AMP. Below the graph is the calculated AMP stimulation values (AMP stimulated/Basal) at the various concentrations of LiRP A1-4.
