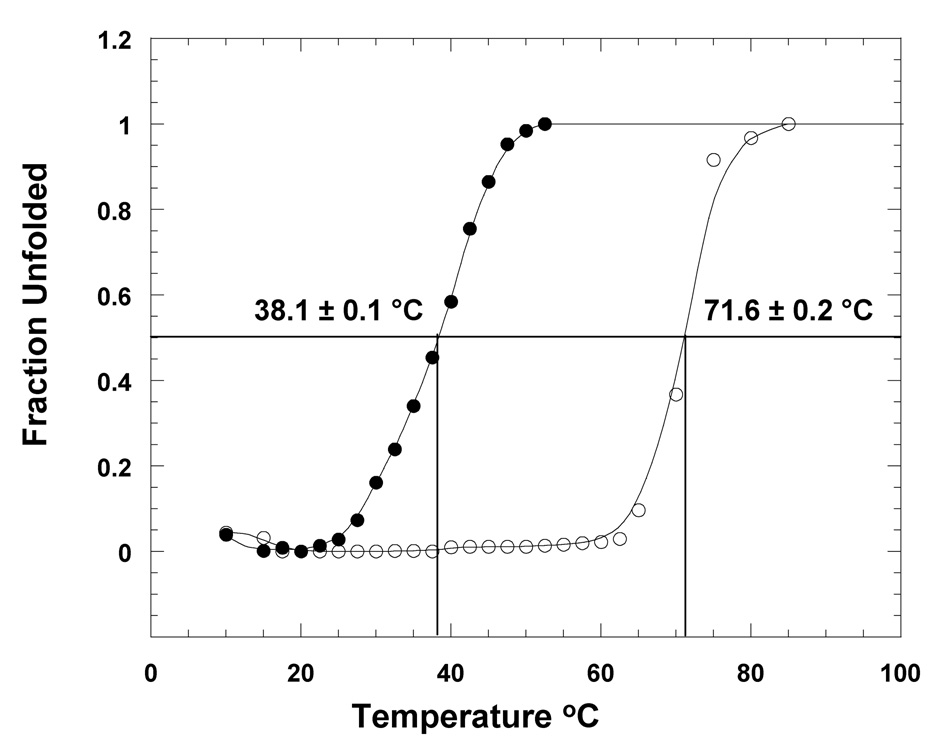
Fig. 4.
Thermal unfolding of apoS100A13 in the absence (open circle) and presence (closed circle) of pS. Thermal unfolding curves were monitored by changes in the intrinsic tryptophan fluorescence at 320 nm. The concentration of the protein used was 25 µg. mL−1. Protein samples were prepared in 10 mM tris (pH 7.5) containing 100 mM NaCl. Protein samples were excited at 280 nm.