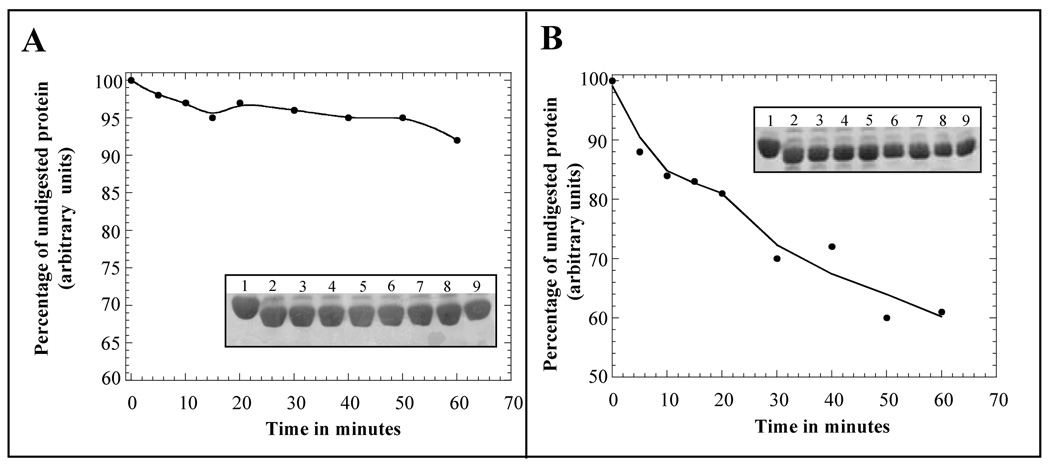
Fig. 5.
Limited trypsin digestion analysis of apoS100A13, in the absence (panel A) and presence (panel B) of pS monitored by SDS-PAGE. The curves represent the denisitometric scans of the S100A13 band (in the SDS-PAGE) at various times of incubation with trypsin. The inset figures show the intensity of the S100A13 band stained with Coomassie blue. The intensity of the S100A13 band not treated with trypsin is assumed as 100%. Proteolytic digesions were performed at an enzyme (trypsin) to substrate (S100A13) molar ratio of 1:1. Trypsin digestion was performed at 25 °C. Gels were destained in 45%:10%:45% methanol-acetic acid-water mixture for 12 hours. Lanes, 1–9, represent the trypsin digestion products after various time periods of incubation of apoS100A13 in the absence (Panel-A) and in the presence (Panel-B) of the pS vesicles. Lane-1, 0 min; lane-2, 5 min; lane-3, 10 min; lane-4, 15 min; lane-5, 20 min; lane-6, 30min; lane-7, 40 min; lane-8, 50 min; and lane-9, 60 min.