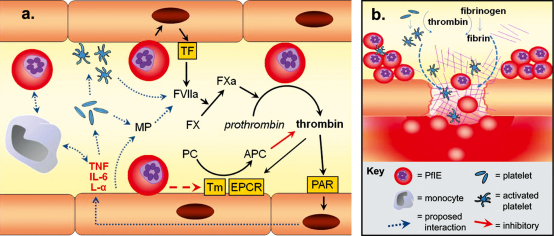
Fig. 1.
Theoretical model for the dysregulation of the coagulation system in cerebral malaria. (a) Early in infection the coagulation system is activated by a combination of the host immune response and parasite-derived protein interactions with host endothelium, and circulating cells. This leads to: (1) the generation of thrombin and intracellular signalling through PAR; (2) the activation and recruitment of platelets and monocytes; (3) the vesiculation of microparticles from the endothelium and platelets; (4) an excessive visibility of TF on the following activated components: endothelium, platelets, monocytes and microparticles. In most patients this system is balanced by the protein C and AT III pathways (latter not pictured here). However, at the most severe end of the spectrum these pathways are impaired by intense sequestration of PfIE within the microvasculature (b). This sets up a potential positive feedback cycle of inflammatory and coagulation events. Thrombin promotes the conversion of fibrinogen to fibrin which adheres to activated platelets to form thrombi and is itself pro-inflammatory. Excessive inflammation at these sites from a combination of inflammatory events and cytokines, unbuffered by anticoagulants, and activation of apoptosis by PfIE contact and TGF-β, leads to endothelial damage and loss of tight junction function. This creates gaps in vessels and, in the context of excessive local consumption of coagulants by the thrombi, leads to microhaemorrhages. L-α: lymphotoxin-α; IL-6: interleukin-6.