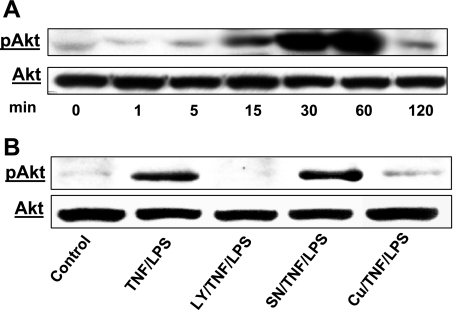
Fig. 2.
Effect of TNF-α/LPS on protein kinase B (Akt) phosphorylation (p) in HIMEC. A: TNF-α/LPS induces Akt phosphorylation in HIMEC. Activation of Akt in HIMEC by TNF-α/LPS was assessed using Western blot analysis. Total cell lysates from cultured HIMEC stimulated with TNF-α (100 IU/ml) and LPS (1 μg/ml) were subjected to SDS-PAGE and immunoblotted with total and phospho-(Ser473)-Akt antibodies. TNF-α/LPS induced phosphorylation of Akt in a time-dependent fashion. TNF-α/LPS stimulation of HIMEC resulted in a strong increase in Akt phosphorylation at 30 min that lasted up to 60 min and declined by 120 min compared with total Akt. Time-matched controls did not show any changes in Akt phosphorylation at any given time points (data not shown). B: pretreatment of HIMEC for 30 min with either curcumin (Cu, 10 μM) or PI 3-kinase inhibitor LY-294002 (LY, 10 μM) before TNF-α/LPS stimulation abolished the Akt phosphorylation, whereas SN-50 (SN, NF-κB inhibitor) did not. Stripping and reprobing of the blot with total Akt antibody demonstrates equivalent protein loading. GSK, glycogen synthase kinase. Data shown are from one of three independent experiments.