Abstract
Overexpression of trefoil factor 2 (TFF2) is associated with increased cell migration, resistance to apoptosis, and possibly increased gastric cancer invasion. Dysregulation of p53 is frequently observed in preneoplastic conditions of the stomach. Here, we investigated the effect of p53 on the expression and function of TFF2 in gastric cancer cell lines. Gene expression was determined by reverse transcription-polymerase chain reaction, and promoter activity was assessed by dual luciferase reporter assays. Apoptosis was detected by flow cytometry, and cell migration was evaluated by the Boyden chamber assay. Exogenous expression of p53 dose dependently inhibited endogenous TFF2 mRNA, protein, and promoter activity and resulted in induction of cell apoptosis and inhibition of cell migration. Downregulation of TFF2 by small interfering RNA sensitized gastric cancer cells to drug-induced p53-dependent apoptosis. Addition of human TFF2 peptide reversed p53-dependent apoptosis and inhibition of cell migration. The p53-responsive element was mapped to an AP-1-like cis-element at −182 bp upstream of the TFF2 transcription start site. Mutation of this AP-1-like element abrogated p53-mediated inhibition of TFF2 promoter activity. Gel shift and chromatin immunoprecipitation assays demonstrated that c-Jun and c-Fos bind to this AP-1-like element. Ectopic expression of c-Jun/c-Fos or p300 or treatment of cells with phorbol 12-myristate 13-acetate (PMA) stimulated endogenous TFF2 mRNA expression and promoter activity, and p53 inhibited the effects of AP-1 and PMA on TFF2. p53 induces cell apoptosis and inhibits cell migration in part by downregulating TFF2 expression through an AP-1-like site, suggesting that TFF2 may be an important downstream target of p53.
Keywords: gastric carcinogenesis, gene regulation
the tumor suppressor protein p53 plays important roles in cell growth, cell cycle and cell migration control, DNA repair, and apoptosis (31). Mutation of p53 is one of the most prevalent genetic alterations in human cancers (16, 22). Gastric cancer arises from a multistep process that involves histological progression from chronic gastritis to gastric atrophy, intestinal metaplasia, dysplasia, and finally gastric cancer (16, 22). Studies show that Helicobacter pylori infection and the associated chronic inflammation leads to p53 mutations, and these mutations may occur in intestinal metaplasia, dysplasia, and gastric cancer (32, 44). The frequency of p53 mutations increases linearly from chronic gastritis to intestinal metaplasia, gastric dysplasia, early gastric cancer, and advanced gastric cancer (15, 27, 29). Thus the data suggest that p53 mutations are common and may even occur at the very earliest stages of gastric carcinogenesis.
Trefoil factor family 2 (TFF2), previously known as spasmolytic polypeptide, functions in wound healing, epithelial restitution, cytoprotection, and anti-inflammatory effect in the gastrointestinal tract by stimulating cell migration and inhibiting apoptosis (6, 14, 17, 21, 41). However, recent evidence indicates that alterations in TFF2 expression are associated with the development of gastric cancer (40). The well-recognized SPEM (spasmolytic polypeptide-expressing metaplasia) lineage, a metaplastic mucous lineage that expresses TFF2, has been detected in up to 91% of cases of gastric carcinoma. TFF2 protein-expressing cells are also upregulated in the stomachs of long-term Helicobacter-infected mice (35, 47) and in patients with H. pylori-associated chronic fundic gastritis and gastric cancer (20, 23, 30). Increased expression of TFF2 protein correlates with the presence of advanced cancer stage, angiogenesis, lymphatic and venous invasion, and poor survival (7, 13, 25). These findings suggest an association between the presence of the SPEM lineage and gastric adenocarcinoma. Thus TFF2 appears to play a role both in normal mucosal cytoprotection and in pathological tumor development in the stomach. Of note, TFF2 RNA and protein expression do not correlate in WT gastric units but in SPEM (35).
Nevertheless, the mechanisms involved in upregulation of TFF2 and its function in the setting of preneoplasia and neoplasia have not been well defined. TFF2 is upregulated early on in metaplasia and later on appears to stimulate cancer cell migration and invasion and to increase angiogenesis (12). Given that mutations in p53 also occur early in preneoplasia and can also promote cell migration and invasion (8, 9), this suggests the possibility of a more direct relationship between p53 mutations and TFF2 expression, including a possible functional link between p53, TFF2, and gastric cancer. Consequently, in this study we investigated the effect of p53 on TFF2 gene expression and function in gastric epithelial cells. Our results show that p53 downregulates TFF2 expression through an AP-1 binding element. This suppression of TFF2 results in increased apoptosis and decreased migration of gastric cancer cells.
MATERIALS AND METHODS
Cell culture and animals.
Human gastric cancer cell lines AGS (ATCC) and MKN-28, MKN-45, MKN-74, and Kato-III (RIKEN, Cell Bank, Ibaraki, Japan) were cultured in DMEM medium supplemented with 10% fetal bovine serum. p53 knockout (p53-KO) mice were obtained from the Jackson Laboratory, and p53R172H knockin mice were a kind gift from Dr. Tyler Jacks (36).
Reporter constructs and PCR mutagenesis.
The full-length human TFF2 promoter (−1429 to +21) was generated through PCR amplification using human genomic DNA (Promega, CA) as a template as previously described (45). Deletion constructs were generated from the full-length fragment, and the promoter fragments were directly cloned into the empty luciferase reporter vector pGL3-basic (Promega) to generate the TFF2 promoter-Luciferase constructs. Transcription factors consensus binding sequences in TFF2 promoter were identified using two software programs “TESS: Transcription Element Search System” and “TRANSFAC.” The TFF2/−219 promoter constructs containing point mutations were generated by using the Site-Directed Mutagenesis kit (Stratagene, TX). For the TFF2/p300-mutant plasmid construct, the primers for the mutant p300 site were forward primer 5′-GAAGAGGTATCACCGAGCAGCCGGAGAGTCACCCTGGCC-3′ and reverse primer 5′-GGCCAGGGTGACTCTCCGGCTGCTCGGTGATACCTCTTC-3′. For the TFF2/AP-1 mutant plasmid construct, the primers for the mutant AP-1 site were forward primer 5′-GTATCACCGAGCAGGGAGAGCTCCACCCTGGCCCGGAAG-3′ and reverse primer 5′-CTTCCGGGCCAGGGTGGAGCTCTCCCTGCTCGGTGATAC-3′; for the TFF2/KLF4-mutant construct, the primers for the mutant KFF4 consensus site were forward primer 5′-cgagcagggagagagtcATTTTGGCCCGGAAGCCTCGCC-3′,5′-GGCGAGGCTTCCGGGCCAAAATGACTCTCTCCCTGCTCG-3′. The italic sequences with underline represent the mutant nucleotides. All wild-type and mutant promoter constructs were confirmed by DNA sequencing.
Cell transfection and luciferase assays.
For reporter gene experiments, cells were transfected with Renilla luciferase reporter and either a TFF2-luciferase construct (TFF2-LUC) or the pGL3-luciferase control construct, in combination with a p53 expression plasmid (gift of B. Vogelstein) or pSUPER.p53 (Oligoengine) or a control plasmid using Superfectin (Qiagen). Luciferase activity was determined by use of the dual luciferase assay system (Promega) and a Monolight 3010 luminometer (Pharmingen).
Generation of stable transfectants expressing the temperature-sensitive mutant p53.
Kato-III cells were transfected with the pCMV-p53Val138 plasmid (gift of Dr. Nobuo Tsuchida) or the control empty pCMV plasmid by using LipofectAMINE 2000 (Life Technologies). Stable transfectants were selected in medium containing Geneticin at 1 mg/ml for 4 wk, and single clones were picked and passaged. The stable clones were further selected by screening for expression of p53 and p21 by RT-PCR and Western blot analysis.
Apoptosis assay.
Gastric cells transfected with the wild-type p53 (p53WT) or control plasmids were cultured with or without 600 nM human glycosylated TFF2 (gift from Dr. Lais Thim) in serum-free medium for 24 and 48 h. In another experiment, gastric cells transfected with TFF2 small interfering RNA (siRNA) or control siRNA (Santa Cruz) were incubated with 10 mM 5-fluorouracil (5-FU, Sigma) or p53R175H or control plasmids for 48 h. Apoptosis was assessed by FACS using the Annixin V-PE Apoptosis Detection Kit (BD Pharmingen, San Diego, CA).
Immunohistochemical staining.
Gastric expression of TFF2 was examined by immunohistochemical staining in 2-mo-old p53 knockout mice, p53175H knockin mice, and wild-type control mice in the same genetic background (C57BL/6). Following death, midline strips removed from the lesser curvature of the stomach were fixed in 10% neutral buffered formalin overnight, processed routinely, and embedded in paraffin. Sections were cut at 5 μm followed by antigen retrieval, achieved by boiling sections for 15 min in 0.01 M citrate buffer, pH 6.0. The rabbit polyclonal TFF2 antibody was raised to the COOH-terminal 16 amino acids (EVPWCFFPQSVEDCHY) from mouse TFF2 but which are highly conserved (e.g., 15 of 16 residues) in the human COOH-terminal peptide (46). Tissue sections were incubated with the TFF2 antibody at a dilution of 1:200 at 4°C overnight in a humidified chamber. Indirect labeling was performed with species-appropriate biotinylated secondary antibodies followed by avidin-biotin complex (ABC)-peroxidase (ABC kit; Vector Laboratories, Burlingame, CA) according to the manufacturer's protocol. 3-Amino-9-ethylcarbazole (Vector Laboratories) was used as chromogen, and slides were counterstained with Mayer's hematoxylin. Stained sections were examined by light microscopy (CX31; Olympus Optical, Cebu, Philippines).
ELISA method.
A sandwich ELISA was developed by using a commercial goat TFF2 antibody anti-TFF2 SP(P-19) (Santa-Cruz Biotechnology, Santa Cruz, CA) and a rabbit anti-TFF2 antibody developed in our laboratory (46). The Santa Cruz goat polyclonal anti-TFF2 recognizes a peptide epitope located within an internal region of human TFF2 and was used as the primary capturing antibody. The rabbit polyclonal antibodies raised against the 16 COOH-terminal amino acids of murine TFF2, which recognizes both mouse and human TFF2 (46), was used as the secondary antibody in the sandwich technique. The wells of a microtiter plate (Nunc-Maxisorp) were coated overnight with 100 μl of capturing antibodies (Santa Cruz Biotechnology) (20 μg/ml in sodium carbonate buffer pH 9.5), blocked with 10% FBS for 1 h, and then washed with PBS containing 0.05% Tween-20. Cell culture supernatant (100 μl) from the study samples was added in triplicate to the antibody-coated wells and incubated overnight at room temperature. After the washing, 100 μl of rabbit polyclonal antibodies (3 μg/ml) were added to the wells for 5 h. After further washing, 100 μl/well of horseradish peroxidase-linked F(ab′)2-conjugated goat anti-rabbit fragment (from donkey) (NA9340V, GE Healthcare, 1:5,000) was added and incubated for 1 h at room temperature. Following further washing, 100 μl of substrate with concentration 0.4 g/l of 3,3′,5,5′-tetramethylbenzidine (TMB Microwell Peroxidase Substrate System, KPL) was added. The reaction was stopped by adding 100 μl of 1 mol/l phosphoric acid to each well. Absorbance was measured photometrically at a wavelength of 450 nm. Positive controls are samples in this assay included recombinant human TFF2, and negative controls included RPMI-1640 medium alone without cells. The concentration of the calibrators ranged from 1 to 100 ng/ml for the TFF2-ELISA. The signal obtained for the lowest calibrator was 1.4–2.3 times the signal obtained for the zero calibrator. On the basis of these results, the detection limits of the assays were judged to be 10 ng.
Cell migration.
Cell migration assays were performed with modified Boyden chambers (8 μm pores, Costar, Cambridge) as described previously (8, 9). Briefly, cells were transiently transfected with the p53WT or control vector and then cultured in the presence of 600 nM TFF2 for 24 h prior to seeding in the upper chamber of the Boyden apparatus; 5 × 104 cells in serum-free media were then added to each upper migration chamber that had previously been coated with fibronectin (Upstate Biotechnology). Medium supplemented with 0.5% BSA (600 μl) was added to the lower chamber. The cells were incubated for 6 h. Nonmigratory cells on the upper membrane surface were removed with a cotton swab. The migratory cells attached to the bottom surface of the membrane were fixed and stained with crystal violet and counted.
Quantitative and semiquantitative polymerase chain reaction.
Total RNA was extracted using TRIzol (Invitrogen). Quantitative and semiquantitative PCR was performed as previously described (27). The sequences of the human TFF2 primers used were as follows: forward 5′-ATGGGACGGCGAGACGCCCA-3′; reverse 5′-TTAGTAATGGCA GTCTTCCACAG-3′. The sequences of the mouse TFF2 primer used were as follows: forward 5′-GCAGTGCTTTGATCTTGGATGC-3′; reverse 5′-TCAGGTTGGAAAAGCAGCAGTT-3′. The sequences of the human p53 primers used were as follows: forward 5′-CCTCCTGGCC CCTGT CATCTT-3′; reverse 5′-GGCGGGGGTGTGGAATCAA-3′. The sequences of the human p21 primers used were as follows: forward 5′-AGCTGAGCCGCGACTGTGATGC-3′, reverse 5′-CAGCCGGCGTTTGGAGTGGTAGAA-3′.
Western blot.
Cells were lysed in lysis buffer. The blots were incubated with primary antibodies overnight and then incubated with a peroxidase-conjugated goat anti-rabbit secondary antibody (Amersham, Piscataway, NJ). The immunoband was visualized by enhanced chemiluminescence (ECL, Amersham, Piscataway, NJ). The p53, p21 and α-tubulin antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA).
Chromatin immunoprecipitation.
Formaldehyde cross-linking and chromatin immunoprecipitation (ChIP) assays were performed as described previously (1). Briefly, gastric cells were incubated with 1% formaldehyde and sonicated into chromatin fragments. Immunoprecipitation was performed with protein G-Sepharose and 8 μg of antibodies to the following: p53 (Ab7; Oncogene Science), c-Jun (06–866; Upstate), c-Fos (sc-52; Santa Cruz). The chromatin solution was precleared by incubation with protein G-Sepharose and incubated with the antibodies overnight at 4°C. PCR reactions were carried out using primers spanning the −182AP-1 site of TFF2 promoter (forward 5′-GAGGATACCTTGCAAGGCTGGA-3′, and reverse 5′-GCACACCAT TGC ACGTGAATGT-3′). The PCR products were analyzed by agarose gel electrophoresis.
Electrophoretic mobility shift assays.
Gel shift assays were performed according to the Lightshift Chemiluminescent EMSA kit manual instructions (Pierce, Rockford, IL). Briefly, nuclear extracts were prepared from AGS cells or Kato III cells and transfected with p53 expression constructs. For EMSAs, the −182TFF2-AP-1 wild-type oligonucleotide, containing the AP-1 binding site-like element located in the hTFF2 promoter region (−195 bp to −176 bp, 5′-CAGGGAGAGAGT CACCCTGGCC-3′), and the −182TFF2-AP-1 mutant oligonucleotide (5′-CAGGGAGATGTCCACCCTGGCC-3′, bold italics with underline indicate the mutant sequences) were used as probes. The complementary single-stranded −182AP-1 probe was first biotin labeled by using terminal deoxynucleotidyl transferase from a biotin 3′-end DNA labeling kit (Pierce) and then reannealed. Nuclear extracts (5 μg) prepared from gastric cancer lines were mixed with the biotinylated probe in the presence of 50 ng/ml of poly(dI-dC), 5% glycerol, with or without 50-fold excess unlabeled probe (cold competitor). For supershift assays, 3 μg of c-Fos and c-Jun antibodies were added into the reaction mixture. The mixtures were loaded onto 6% DNA retardation gels (Invitrogen). The DNA was cross-linked, and biotin-labeled DNA was detected by chemiluminescence using streptavidin-horseradish peroxidase conjugate (Pierce Biotechnologies, Rockford, IL).
Statistical analysis.
Data are presented throughout as means ± SE. Statistically significant differences (P < 0.05) were decided by the Student t-test.
RESULTS
p53 downregulates endogenous TFF2 expression in gastric cancer cell lines.
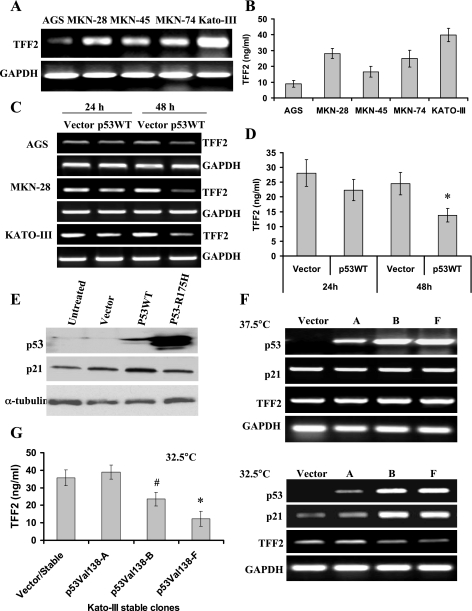
At first we analyzed endogenous expression of the TFF2 gene in gastric cell lines. Five gastric cancer cell lines with variable p53 expression status were used: AGS, MKN-45, and NKN-74 (wild-type p53); MKN-28 (mutant p53); and Kato-III (p53 deletion) (48). TFF2 mRNA was present in all gastric cancer cell lines tested. AGS cells had a relatively low level of TFF2 mRNA, whereas Kato-III cells expressed the highest level of TFF2 mRNA (Fig. 1A). Previous studies have indicated that TFF2 protein is not detectable in gastric cancer cell lines by Western blot (3, 4, 45). However, since TFF2 is a secreted peptide (10, 49), we developed a sandwich ELISA technique that could detect low levels of TFF2 peptide and assayed the supernatant of cultured gastric cancer cells. Consistent with the RT-PCR results, KATO-III cells secreted the highest level of TFF2 peptide, whereas AGS cells secreted a lowest level of TFF2 peptide (Fig. 1B). Transient expression of wild-type p53 (p53WT) downregulated TFF2 mRNA expression in AGS, MKN-28, and Kato-III cells as measured by RT-PCR (Fig. 1C) and real-time PCR (data not shown). Overexpression of p53WT also inhibited the secretion of TFF2 peptide in gastric cancer cells (Fig. 1D). A moderate level of p53 protein and a high level of p21 protein were detected in Kato-III cells following transfection with p53WT expression construct (Fig. 1E), indicating that p53WT specifically inhibited the expression of TFF2.
Fig. 1.
Exogenous expression of p53 downregulates the level of trefoil factor 2 (TFF2) in gastric cancer cells. A: TFF2 mRNA expression levels in gastric cancer cell lines as determined by semiquantitative RT-PCR. B: the levels of TFF2 peptide in the supernatant of gastric cancer cell lines as measured by ELISA methods. C: p53 downregulates the level of TFF2 mRNA. Gastric cancer cells were transfected with a p53 overexpression construct or control vector for 24 and 48 h. TFF2 mRNA expression was determined by RT-PCR. D: p53WT decreases the level of TFF2 peptide. Kato-III cells were transfected with p53WT plasmid or control vector for 24 and 48 h. Cell supernatant was collected and TFF2 level was measured by ELISA methods (*P < 0.05 vs. control vector). E: protein expression in Kato-III gastric cancer cells. Kato-III cells were transfected with pCMV-p53-WT or pCMV-p53R175H plasmids for 24 h. Expression of p53 and p21 proteins was assessed by Western blot. F: temperature-sensitive mutant p53val138 downregulates TFF2 mRNA expression. Stable Kato-III/p53val138 transfectants A, B, and F and 1 control vector transfectant were selected and cultured at 37.5 or 32.5°C, respectively. Messenger RNA expression of p53, p21, and TFF2 were determined by RT-PCR. G: temperature-sensitive mutant p53val138 decreases level of TFF2 peptide. Stable Kato-III transfectants were cultured at 32.5°C for 48 h. Cell supernatant was collected and TFF2 level was measured by ELISA method (*P < 0.05, #P < 0.05 vs. control vector).
We generated several Kato-III stable cell clones expressing the temperature-sensitive mutant p53val138 (Fig. 1F). These stable clones expressed a nonfunctional p53 protein when cultured at 37.5°C (nonpermissive temperature) and expressed high levels of functional p53 and p21 mRNA (Fig. 1F) and protein (data not shown) when cultured at 32.5°C (permissive temperature). In the p53val138 stable clones B and F, TFF2 mRNA expression was not significantly altered when cells were cultured at 37.5°C, whereas at 32.5°C TFF2 mRNA expression (Fig. 1F) and TFF2 protein (Fig. 1G) was significantly decreased, indicating that the temperature-sensitive p53 mutant inhibits TFF2 expression.
Interference with p53 function increases TFF2 gene expression.
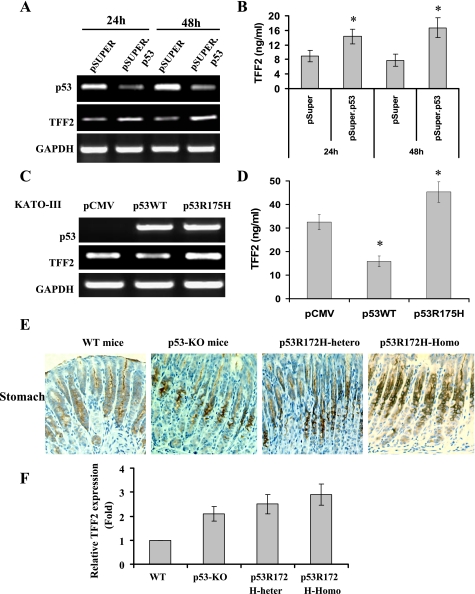
Next, we examined whether interference with p53 function would modulate expression of TFF2. The pSUPER.p53 siRNA significantly inhibited p53 expression and increased TFF2 mRNA expression in AGS cells confirmed by RT-PCR (Fig. 2A) and real-time RT-PCR (data not shown). The pSUPER.p53 siRNA also significantly increased TFF2 protein secretion (Fig. 2B). The gain-of-function p53R175H mutant, in contrast, increased expression of TFF2 mRNA (Fig. 2C) and TFF2 protein level (Fig. 2D). To determine whether p53 deficiency influences in vivo expression of TFF2, we examined the expression of TFF2 in 2-mo-old homozygous p53-KO mice, heterozygous p53R172H, and homozygous p53R172H knockin mice. Immunohistochemical staining showed that TFF2 protein expression was increased in the gastric fundus of p53-KO mice, heterozygous p53R172H, and homozygous p53R172H knockin mice compared with the C57BL/6 controls (Fig. 2D). Expression of TFF2 protein was localized primarily to gastric mucous neck cells of the corpus in all murine strains (Fig. 2E). Real time RT-PCR indicated that TFF2 mRNA expression was upregulated in the stomachs of p53-KO, heterozygous p53R172H mice, and homozygous p53R172H mice compared with age-matched control animals (Fig. 2F). These results suggest that interference with p53 function increases TFF2 expression both in vitro and in vivo.
Fig. 2.
Interference with p53 function upregulates TFF2 gene expression. A: knockdown of p53 mRNA expression by small interfering RNA (siRNA) increases the expression of TFF2 mRNA. AGS cells were transfected with pSUPER.p53 or pSUPER control vector for 24 and 48 h. Messenger RNA expression was determined by RT-PCR. B: knockdown of p53 expression increases the level of TFF2 protein. AGS cells were transfected with pSUPER.p53 or pSUPER control vector for 24 and 48 h. Cell supernatant was collected and TFF2 level was measured by ELISA. (*P < 0.05 vs. pSUPER control vector). C: p53 mutation upregulates TFF2 mRNA expression. KATO-III cells were transfected with p53WT or p53R175H mutant plasmids for 48 h. Messenger RNA expression was determined by RT-PCR. D: p53 mutation increases level of TFF2 peptide. KATO-III cells were transfected with p53WT or p53R175H mutant plasmids for 48 h. Cell supernatant was collected and TFF2 level was measured by ELISA (*P < 0.05 vs. control vector). E: increased expression of TFF2 protein in p53-knockout (p53-KO) mice, p53R172H heterozygous mice, and p53R172H homozygous mice. Representative immunohistochemical staining for TFF2 in indicated knockout mice, knockin mice, and control wild-type (WT) mice (original magnification ×400). F: upregulation of TFF2 mRNA expression in stomach tissues from p53-KO mice, p53R172H heterozygous mice, and p53R172H homozygous mice. Messenger RNA was prepared from 2-mo-old p53-KO mice and p53R172H mice and age- and gender-matched control mice (5 mice/group) (*P < 0.05. vs. WT mice). The levels of TFF2 mRNA were determined by real-time PCR and expressed as means ± SD.
p53 inhibits TFF2 transcription in gastric cancer cells.
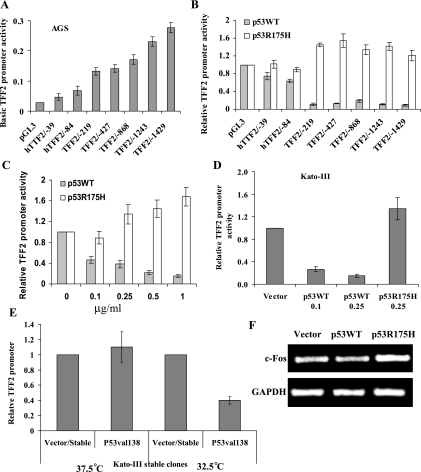
Next, we investigated the mechanisms by which p53 regulates TFF2 gene expression, using a series of truncated TFF2 promoter-luciferase reporter constructs. The basal activity of the TFF2 promoter constructs showed a gradual increase from the −39-bp truncation to the full-length 1423-bp construct (Fig. 3A). p53 inhibited the activity of a series of TFF2 promoters. The strongest inhibition by p53 could be localized to the −84 to −219-bp region upstream of TFF2 transcription at start site (Fig. 3B). p53 inhibited the TFF2 promoter activity in a dose-dependent manner in AGS and Kato-III cells (Fig. 3, C and D) but increased p21 promoter activity (data not shown). The temperature-sensitive p53 mutant at the permissive temperature inhibited TFF2 promoter activity, whereas promoter activity was unchanged at 37.5°C (Fig. 3E). Furthermore, the gain-of-function p53R175H mutant increased TFF2 promoter activity in AGS and Kato-III cells (Fig. 3, B–D). These data demonstrate the specific inhibitory effect of p53WT on TFF2 promoter activity. Moreover, p53R175H upregulated the expression of c-Fos in Kato-III cells, consistent with previous report that p53R175H increased the expression of c-Fos in p53-null H1299 cells (39) (Fig. 3F). Taken together, these data suggest that p53WT inhibits TFF2 transcription in gastric cancer cells.
Fig. 3.
p53 inhibits TFF2 transcription in gastric cancer cells. A: basal activity of TFF2 promoter-luciferase constructs in untreated AGS cells. The data represent means ± SE derived from a minimum of 3 separate experiments. B: mapping p53-responsive regions in the TFF2 promoter. AGS cells were cotransfected with the indicated TFF2-promoter plasmids and Renilla luciferase vector with 0.5 μg of pCMV-p53, p53R175H, or pCMV empty plasmids. The data presented represent the average relative luciferase activity (fold) compared with the empty vector in 3 independent experiments. C and D: p53 dose dependently inhibits the activity of the TFF2 promoter. AGS cells (C) and Kato-III cells (D) were cotransfected with the TFF2/−1423 promoter construct and Renilla luciferase vector with pCMV-p53, p53R175H, or pCMV empty plasmids at the indicated dose. The amount of transfected DNA was maintained at a constant level by adjusting the amount of the pCMV empty vector. E: temperature-sensitive p53val138 mutant inhibits TFF2 transcription in cells cultured at 32.5°C. Kato-III/p53val138 stable transfectant B and control vector transfectant were transfected with TFF2/−1243 promoter reporter construct and Renilla luciferase vector. The stable transfectants were cultured at 37.5 and 32.5°C, respectively. Luciferase activity was determined by using the dual luciferase assay system 48 h after transfection.
An AP-1-like element is required for p53-mediated inhibition of TFF2 promoter activity.
To further map the p53-responsive element, we generated two additional TFF2 deletion constructs: TFF2/−134 and TFF2/−175. Luciferase assays revealed that deletion to 175 bp abrogated the p53 inhibitory effect, thus localizing the p53-responsive element to the −175- to −219-bp region upstream of the TFF2 transcription start site (Fig. 4A). This region did not contain any obvious consensus p53 binding site, suggesting that the repressive effect of p53 on TFF2 transcription was likely not mediated through direct binding by p53 to its cognate DNA sequences. Computer software-assisted analysis (TESS and RANSFAC) predicted three overlapping transcription factor consensus DNA binding sequences (Fig. 4B). These represent putative p300, AP-1, and KLF4 consensus binding sites, with the DNA sequences of 5′-GGGAGAG-3′, 5′-AGAGTCA-3′, and 5′-CACCC-3′, respectively (Fig. 4B). Each was mutated separately in the TFF2/−219 construct. Mutation of the putative AP-1 binding element significantly increased basal TFF2/−219 promoter activity in AGS cells (Fig. 4C) but not in Kato-III cells (Fig. 4D). There were no significant changes in basal activity of the TFF2/−219 promoter construct with mutation of putative p300 or KLF4 sites (Fig. 4, C and D). Since AGS cells express endogenous p53WT, the mutated AP-1 site likely abrogated the inhibitory effect by endogenous p53 on TFF2 promoter activity. Moreover, mutation of the AP-1-like element abolished the inhibitory effect by exogenous p53 on TFF2 promoter activity in Kato-III cells, whereas the mutation of p300 and KLF4 sites did not affect the inhibitory effect of p53 on TFF2 promoter activity (Fig. 4E).
Fig. 4.
AP-1-like element mediates p53-dependent inhibition of TFF2 promoter activity. A: deletion analysis localizes the p53-responsive region to the −175 to −219-bp region of the human TFF2 promoter in AGS cells. B: diagram of the p53-responsive element and location of mutations in TFF2 promoter in the −175 to −219-bp region. There are 3 transcription factor consensus sites (p300, AP-1, and KLF4) that are labeled and the corresponding mutated sequences below are underlined. C and D: mutation of the AP-1-like element abolishes p53-dependent inhibition of the TFF2 promoter. AGS cells (C) and Kato-III cells (D) were cotransfected with the indicated TFF2 promoter reporter construct along with the p53 expression plasmid or control vector. E: mutation of the AP-1-like element abolishes inhibition by exogenous p53 of TFF2 promoter activity in Kato-III cells. Kato-III cells were cotransfected with the indicated TFF2-promoter reporter construct and Renilla luciferase vector along with pCMV-p53 or pCMV empty vector. The data shown represent means ± SD of 3 independent experiments.
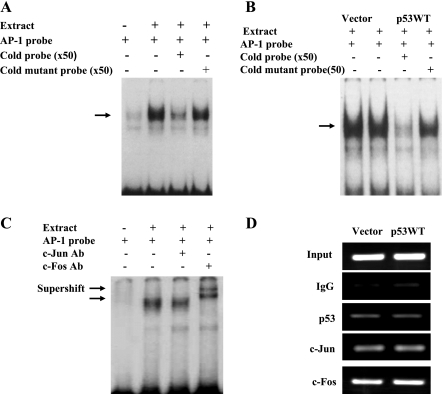
The transcription factors c-Jun and c-Fos bind to the −182AP-1 site in the TFF2 promoter.
We next investigated whether p53 might affect AP-1 transcription factor binding to this −181AP-1 site. We performed gel mobility shift assays using an oligo probe encompassing the −182AP-1 site. EMSA showed that AGS nuclear extracts formed a specific complex with the labeled probe and that binding was efficiently competed for by cold wild-type −181AP-1 probe, but not by cold mutant AP-1 probe (Fig. 5A). Similar DNA-protein complexes were obtained using nuclear extracts from Kato-III cells transfected with either p53WT or empty vector (Fig. 5B). Thus it appears that p53WT expression does not alter factor binding to the −182AP-1 element. To investigate whether −182AP-1 site is a target of the AP-1 protein complex, we performed supershift experiments with nuclear extracts. Addition of c-Fos antibody to the binding reaction induced a marked supershift, whereas the c-Jun antibody resulted in a weak retarded band (Fig. 5C). To obtain direct evidence that AP-1 proteins c-Jun and c-Fos bind to the −181 AP-1 sites in the TFF2 promoter, we performed ChIP assays to detect formation of AP-1 binding complex with c-Jun and c-Fos antibody in Kato-III cells transfected with p53. The ChIP assay revealed binding of c-Jun and c-Fos to the −182AP-1 element of the TFF2 promoter, but overexpression of p53 did not influence the binding (Fig. 5D).
Fig. 5.
c-Jun/c-Fos bind to the −182AP-1-like element in the TFF2 promoter. A: binding of nuclear factors to the AP-1 element in the TFF2 promoter. EMSA was performed by using AGS nuclear proteins with biotin-labeled WT −181AP-1 consensus probe as indicated in the presence or absence of 50-fold molar excess of unlabeled WT-181AP-1 or mutated-181AP-1 probes. B: p53 does not modulate c-Jun/ c-Fos binding to the AP-1-like element. Nuclear proteins extracted from KATO cells transfected with either p53WT or pCMV were incubated with biotin-labeled −181AP-1 probe in the presence or absence of 50-fold molar excess of unlabeled WT or mutated −181AP-1 probes. C: binding of c-Jun and c-Fos to the −181AP-1 element in the TFF2 promoter as measured by EMSA. AGS cell nuclear proteins were incubated with biotin-labeled −181AP-1 probe in the presence or absence of the indicated antibodies. D: chromatin immunoprecipitation (ChIP) assays confirm c-Jun and c-Fos binding to TFF2 promoter. KATO cells were transfected with p53WT or control pCMV for 48 h. Antibodies against p53, c-Jun, c-Fos, and control IgG were used to precipitate DNA-protein complexes. Purified precipitated DNA-protein complexes were used as a template for PCR to amplify a fragment containing the −182AP-1 site of the TFF2 promoter.
p53 inhibits PMA- and c-Jun/c-Fos-induced TTF2 expression and promoter activity.
To confirm a role for AP-1 in the regulation of p53-dependent TFF2 promoter activity, we investigated whether components of AP-1 directly activated the TFF2 promoter. Cotransfection of the AP-1 components c-Jun/c-Fos activated threefold the TFF2/−219 promoter activity in AGS cells, and p53 significantly inhibited this response (Fig. 6A). Mutation of the AP-1 binding site abrogated c-Jun/c-Fos-dependent activation and p53-dependent inhibition of the TFF2 promoter (Fig. 6B). PMA, a tumor promoter and activator of AP-1, stimulated endogenous TFF2 mRNA expression and TFF2 promoter activity, whereas p53 inhibited the effect of PMA on TFF2 (Fig. 6, C and D). Mutation of the AP-1 binding site also abrogated PMA-dependent activation and p53-dependent inhibition of TFF2 promoter activity (data not shown). These data confirm that AP-1 directly activates the TFF2 promoter, and the AP-1-like binding element is required for both activation and repression of TFF2 gene expression.
Fig. 6.
p53 inhibits c-Jun/c-Fos- and phorbol 12-myristate 13-acetate (PMA)-induced TFF2 gene expression and TFF2 promoter activity. A: AP-1 components (c-Jun/c-Fos) stimulate TFF2 promoter activity. AGS cells were cotransfected with the TFF2/−219 promoter construct, pGL3 empty vector, and Renilla luciferase vector with pCMV-c-Fos/pRSV-c-Jun plasmids and/or p53WT plasmid. Luciferase activity was measured 48 h after transfection. B: mutation of the AP-1-like element abrogates AP-1-mediated activation and p53-dependent inhibition of the TFF2 promoter. AGS cells were cotransfected with the TFF2/−219-AP1-M promoter plasmid or the pGL3 empty vector with pCMV-c-Fos/pCMV-c-Jun plasmids. The data presented represent the average luciferase activity (fold change) compared with the pCMV empty vector from 3 independent experiments. C: p53 inhibits PMA-induced TFF2 mRNA expression. AGS cells were transfected with the p53WT or control vector in the presence or absence of 10 ng/ml PMA for 48 h. Messenger RNA expression was determined by RT-PCR. D: p53 inhibits PMA-induced activation of the TFF2 promoter. AGS cells were cotransfected with the TFF2/−219 promoter plasmid along with the p53WT plasmid or control plasmid in the presence of 10 ng/ml PMA for 48 h. Luciferase activity is expressed as activity relative to that of the empty vector. E: p53 inhibits p300-induced activation of the TFF2 promoter. AGS cells were cotransfected with the TFF2/−219 or TFF2/−219-AP-1-M promoter plasmids along with the p300, p53WT, or control plasmids in the presence of for 48 h. Luciferase activity is expressed as activity relative to that of the empty vector.
p300 involved in the p53 transcriptional regulation of TFF2.
Our studies above indicate that p53-dependent inhibition of TFF2 gene expression is mediated through AP-1 but likely involves both an effect on AP-1 binding and an effect independent of AP-1 binding. To further study the potential mechanism of p53-dependent inhibition of TFF2, we investigated whether p53 interferes with interactions between AP-1 and other components of the transcriptional complex. The coactivator protein p300 has been implicated in p53-mediated transcriptional inhibition (2). To test the role of p300 in the p53 transcriptional inhibition of TFF2, AGS cells were cotransfected with the TFF2/−219 or TFF2/−219-AP-1-M report plasmids and pCMV-p300 plasmid. The results from these studies showed that the p300 plasmid upregulated TFF2 promoter activity and that p53 inhibited p300-mediated activation of the TFF2 promoter (Fig. 6E). Since p53-mediated downregulation of TFF2 was AP-1 dependent, we examined whether p300 activation of the TFF2 promoter and p53 inhibition of p300-mediated activation of TFF2 promoter were AP-1 dependent. As shown in Fig. 6E, mutation of the −219AP-1 site significantly decreased the activation of the TFF2 promoter by p300, suggesting that p300 is a coactivator of AP-1 in the transcriptional regulation of TFF2. More importantly, p53-dependent inhibition of p300-mediated activation of the TFF2 promoter was completely abolished after the −219AP-1 site was mutated (Fig. 6E), confirming roles for both p300 and AP-1 in the p53 transcriptional regulation of TFF2.
p53-dependent regulation of apoptosis and cell migration are mediated by suppression of TFF2.
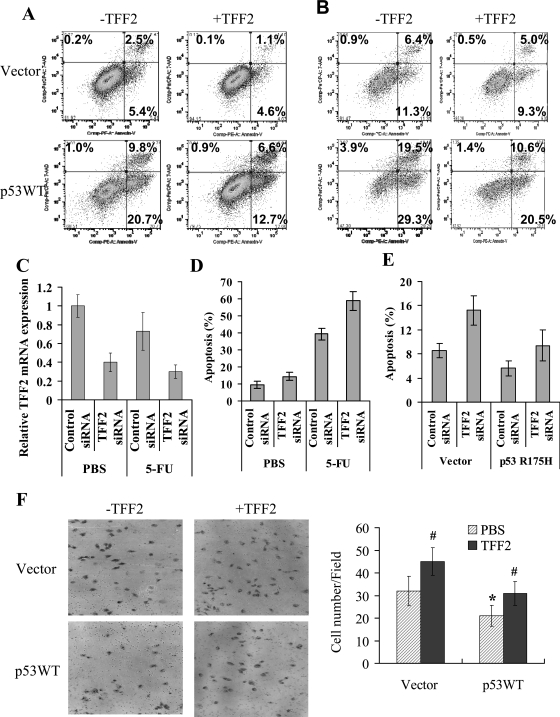
Finally, we investigated the functional effect of p53 downregulation on TFF2 expression. p53 is an known as an inducer of cell apoptosis (18), whereas TFF2 has been reported to inhibit cell apoptosis (41). Thus we investigated a possible functional relationship between p53 and TFF2 with respect to programmed cell death. Transient overexpression of p53WT significantly inhibited TFF2 expression and induced cell apoptosis in Kato-III cells in a time-dependent manner (Figs. 1B and 7, A and B). Addition of exogenous human TFF2 peptide reduced p53-induced apoptosis (Fig. 7, A and B). The temperature-sensitive p53 mutant induced cellular apoptosis and addition of recombinant TFF2 peptide reduced cell apoptosis at 32.5°C culture (data not shown). To evaluate the role of TFF2 in p53-dependent apoptosis, the MKN-74 cells expressing wild-type p53 were treated with TFF2 siRNA combination with 5-FU, a chemotherapeutic drug that induces apoptosis in p53-dependent manner. The results showed that TFF2 siRNA treatment downregulated expression of TFF2 (Fig. 7C) and sensitized MKN-74 cells to serum withdrawal- and 5-FU-induced apoptosis (Fig. 7, D and E). In addition, p53R175H partially protected cells from apoptosis induced by serum withdrawal and downregulation of TFF2 (Fig. 7E). Taken together, the data suggest that p53-induced apoptosis is mediated in part by downregulation of TFF2 expression.
Fig. 7.
p53-dependent apoptosis and cell migration are mediated by modulation of TFF2 expression. A and B: effects of exogenous TFF2 on p53-induced apoptosis in gastric cancer cells. Kato-III cells were transfected with p53WT and control vector in absence and presence of 600 nM TFF2 for 24 h (A) and 48 h (B). Cell apoptosis was detected by FACS using Annexin V-PE apoptosis kit. Representative FACS blot are shown and the data shown represent means ± SE of 3 independent experiments. C and D: downregulation of TFF2 expression sensitized gastric cancer to drug-induced apoptosis. MKN-74 cells were transfected with TFF2 siRNA and control siRNA and then incubated with and without 10 mM 5-fluorouracil (5-FU) for 48 h. Cells were harvested. TFF2 expression was detected by real-time PCR (C) and apoptosis were measured by FACS. The data shown represent means ± SD of 3 independent experiments. E: p53R175H protects gastric cancer cells from apoptosis induced by downregulation of TFF2. MKN-74 cells were cotransfected with TFF2 siRNA or control siRNA in combination with p53R175H or control plasmids for 48 h. Cells were harvested and apoptosis was measured by FACS. The data shown represent means ± SD of 3 independent experiments. F: addition of recombinant TFF2 reverses p53-induced inhibition of cell migration in gastric cancer cells. Transient Kato-III cells transfected with P53WT and control vector in the presence and absence of 600 nM TFF2 were seeded on a Transwell filter inset in a modified Boyden chamber. The migrating cells were fixed on the filter after 6 h, stained, and counted under the microscope. Representative microphotographs are shown and the data represent means ± SE of 3 independent experiments (*P < 0.05 vs. Vector; #P < 0.05 vs. PBS group).
Given that p53 suppresses cell migration (38), whereas TFF2 stimulates cell migration (5, 28), we next examined whether downregulation of TFF2 contributes to the migratory inhibition by p53. We found that transient overexpression of p53WT reduced cell migration, whereas the addition of recombinant TFF2 completely restored the migration defect (Fig. 7F), suggesting that p53 inhibits cell migration in a large part by downregulation of TFF2 expression. Moreover, transient expression of p53R175H increase cell migration in AGS cells (data not shown), indicating that dominant negative p53 mutant may promote cell migration in gastric cancer cells, consistent with previous reports (8, 9).
DISCUSSION
In this study, we show that p53 downregulates TFF2 gene expression both in vitro and in vivo and that suppression of TFF2 results in the induction of apoptosis and inhibition of cell migration. The importance of TFF2 in mediating these effects was supported by the finding that recombinant TFF2 restored the apoptotic and migratory phenotype in cells expressing WTp53 and that downregulation of TFF2 sensitized gastric cancer cells to p53-dependent chemotherapeutic drug-induced apoptosis. Previous studies have suggested that TFF2 can promote cell migration and branch cell morphogenesis (11) and can inhibit apoptosis in cancer cell lines (42). It is also known that the control of cell migration and induction of apoptosis are important tumor suppressor functions of p53 (38). Our data suggest that p53 represses TFF2 transcription through an AP-1 element, located on the TFF2 promoter, that binds c-Fos/c-Jun independent of AP-1 binding. Therefore, upregulation of TFF2 may be an important consequence resulting from the loss of p53 function.
TFF2 is upregulated in diverse gastrointestinal pathological conditions in both human and mouse (31). Interestingly, there is a tight correlation between TFF2 RNA and protein expression in gastric metaplasia (SPEM), in contrast to a distinct expression of TFF2 RNA in preneck cells and TFF2 protein expression that is found in more mature mucus neck cells (35). The reason for the low levels of TFF2 protein expression in human gastric cancer cells remains unknown but seems likely to involve posttranscriptional regulatory mechanisms (such as microRNA).
Studies from our group have shown that expression of TFF2 can be transcriptionally regulated through a spasmolytic polypeptide-responsive element (3), C/EBP consensus-binding site (4), and gastrin-responsive cis-acting elements (46). It has been reported that p53 represses the expression of antiapoptotic and prometastatic genes, which may involve direct binding of p53 to promoters, or interaction of p53 with other transcription factors such as SP-1, AP-1, and p300, or recruitment of histone deacetylase complexes containing mSin3a (26, 44). p53 downregulates MMP-1 by disrupting the interaction between AP-1 and the coactivator p300 (44) and inhibits PMA- and IL-1β-induced MMP-1 gene transcription through a p53-binding site-independent mechanism (43). Additional studies have shown that p53-mediated repression of CXCR4 requires a cyclic AMP/AP-1 (CRE/AP-1)-like element in the CXCR4 promoter, but p53 does not affect the binding of c-Jun and ATF-1 to the CRE/AP-1 element (33). Thus cross talk between AP-1 family members and p53 is an important mechanism by which p53 negatively regulates expression of some genes.
In this study, we show that p53 inhibited the TFF2 promoter through an AP-1 binding element-dependent mechanism, a new regulatory mechanism of TFF2 expression. Since p53 equally inhibited endogenous AP-1-mediated and ectopically expressed c-Jun/c-Fos-mediated induction of TFF2/−219-luciferase, p53 inhibition of TFF2 expression appears not to be solely due to the downregulation of genes that compose the AP-1 transcription factor. In addition, gel shift and ChIP assays showed that p53 did not directly affect the binding of AP-1 components (c-Fos and c-Jun) to the 182-AP-1 element in the TFF2 promoter. Thus it is conceivable that p53 possibly interferes with the interaction between the transcription factor AP-1 and the basal transcription complex, which may involve sequestering of the coactivator p300/CBP. p300/CBP has also been implicated in p53-mediated transcriptional inhibition (2, 37, 41, 44). Interestingly, there is in fact a p300 consensus site, which overlaps with the AP-1 element in the TFF2 promoter. Although a mutation in the p300 site did not abrogate p53 inhibition of TFF2, this does not exclude the possibility that p300 cooperates with AP-1 in the activation of TFF2 transcription. In fact, our results have shown that p300 directly activates TFF2 promoter activity and that p53 inhibits p300-dependent activation of the TFF2 promoter. An AP-1 binding site mutant abolished p53 inhibition of the TFF2 promoter induced by p300. These results suggest that p300 is involved in TFF2 transcription in cooperation with AP-1. Thus p53 may sequester p300/CBP away from the TFF2 promoter and reduce interactions between c-Fos/c-Jun and p300, resulting in the inhibition of TFF2.
Our results also revealed that AP-1 and PMA can directly activate expression of TFF2 and that p53 inhibits AP-1 and PMA-mediated activation of TFF2 in gastric cancer cells. This is consistent with a previous report that PMA upregulates the expression of TFF2 in A549 lung cancer cells (24). The transcription factor AP-1 is composed of homodimers or heterodimers formed between Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra-1, and Fra-2) that bind to a consensus site TGA (G/C) TCA (11). AP-1 is involved in numerous important biological processes including cell growth, apoptosis, and cell transformation. AP-1 also regulates genes that are required for tumor invasion and metastasis such as MMPs and CXCR4 (32, 41). Our results confirm that TFF2 is a downstream target of AP-1.
Tumor-associated mutants of the p53 tumor suppressor protein exert biological activities compatible with an oncogenic gain of function. One study has shown that mutant p53R175H induces EGR-1 by physical association with the EGR-1 promoter, providing a significant gain of function (50). In the present study, we demonstrated that p53R175H is capable of inducing TFF2 promoter activity. p53 mutation may inhibit apoptosis and increase cell migration due to its effects on upregulation of TFF2. This is consistent with a recent report that the p53R175H mutant upregulates the expression of the hPar1 gene, resulting in enhanced prostate cancer cell invasion in a p53-null mouse model (39). It remains unclear precisely how the p53 mutants interact with their target promoters. However, microarray analysis has shown that p53R175H can upregulate c-Fos expression (23 fold) (50). In the present study, we show that c-Fos can upregulate TFF2 expression and promoter activity and that p53WT inhibited c-Fos-induced TFF2 expression. Thus the mutant p53R175H enhances TFF2 promoter activity at least in part through regulation of c-Fos expression and activity. These data provide a possible functional link between p53 mutation and the increased expression of TFF2 during the development of gastric cancer and strongly suggest that some of p53's effects may be related to altered TFF2 expression. It has been reported that cell migration and wound healing are faster in p53 mutant and p53-KO cells and mice than in control cells and mice (18, 34), and recent studies have reported that the p53 dominant-negative mutant promote migration and invasion of cancer cells (8, 9). In addition, alterations in the production of mutant p53 and TFF2 have been correlated with the malignant behavior of human gastric cancer cells. The finding that p53 downregulates TFF2 transcription through an AP-1 element provides a mechanism by which p53 mutation can increase TFF2 expression in gastric cancer cells. The p53-dependent inhibition of PMA- and c-Jun/c-Fos-mediated TFF2 activation supports the hypothesis that dysregulation of p53 allows oncogenic or inflammation-stimulated induction of TFF2. Taken together, these data suggest that TFF2 may be an important downstream target of p53.
GRANTS
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Grant (R01 DK58889-01) to T. C. Wang.
Acknowledgments
We thank Dr. Bert Vogelstein for providing the wild-type p53 and p53R175H expression plasmids and Dr. Nobuo Tsuchida Moshe Oren for the pCMV-p53Val138 plasmid. We thank Dr. Tyler Jacks for the generous gift of the p53R172H knockin mice. We acknowledge Dr. Lars Thim for the kind gift of human recombinant TFF2 protein.
REFERENCES
- 1.Ai W, Zheng H, Yang X, Liu Y, Wang TC. Tip60 functions as a potential corepressor of KLF4 in regulation of HDC promoter activity. Nucleic Acids Res 35: 6137–6149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/C BP in p53-dependent signal pathways. Cell 89: 1175–1184, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bulitta CJ, Fleming JV, Raychowdhury R, Taupin D, Rosenberg I, Wang TC. Autoinduction of the trefoil factor 2 (TFF2) promoter requires an upstream cis-acting element. Biochem Biophys Res Commun 293: 366–374, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Chi AL, Lim S, Wang TC. Characterization of a CCAAT-enhancer element of trefoil factor family 2 (TFF2) promoter in MCF-7 cells. Peptides 25: 839–847, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Chwieralski CE, Schnurra I, Thim L, Hoffmann W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am J Respir Cell Mol Biol 31: 528–537, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cook GA, Familari M, Thim L, Giraud AS. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett 456: 155–159, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Dhar DK, Wang TC, Maruyama R, Udagawa J, Kubota H, Fuji T, Tachibana M, Ono T, Otani H, Nagasue N. Expression of cytoplasmic TFF2 is a marker of tumor metastasis and negative prognostic factor in gastric cancer. Lab Invest 83: 1343–1352, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ, Banerjee SK. Gain of oncogenic function of p53 mutants induces invasive phenotypes in human breast cancer cells by silencing CCN5/WISP-2. Cancer Res 68: 4580–4587, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dong P, Tada M, Hamada J, Nakamura A, Moriuchi T, Sakuragi N. p53 dominant-negative mutant R273H promotes invasion and migration of human endometrial cancer HHUA cells. Clin Exp Metastasis 24: 471–483, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem 284: 3650–3662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Emami S, Le Floch N, Bruyneel E, Thim L, May F, Westley B, Rio M, Mareel M, Gespach C. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J 15: 351–361, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Emami S, Rodrigues S, Rodrigue CM, Le Floch N, Rivat C, Attoub S, Bruyneel E, Gespach C. Trefoil factor family (TFF) peptides and cancer progression. Peptides 25: 885–898, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest 109: 193–204, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer 2: 8–14, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A. TP53 and gastric carcinoma: a review. Hum Mutat 21: 258–270, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, Jones EK, Wang TC. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 × Sv129 Helicobacter pylori-infected mice. Am J Pathol 171: 1520–1528, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene 22: 9030–9040, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Guo F, Zheng Y. Rho family GTPases cooperate with p53 deletion to promote primary mouse embryonic fibroblast cell invasion. Oncogene 23: 5577–5585, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, Oddsdottir M, Magnusson J, Lee JR, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci 48: 431–441, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology 105: 1110–1116, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 253: 49–53, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Hu GY, Yu BP, Dong WG, Li MQ, Yu JP, Luo HS, Rang ZX. Expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol 9: 910–914, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang W, Nielsen O, Fenger C, Madsen J, Hansen S, Tornoe I, Eggleton P, Reid KB, Holmskov U. The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester. Clin Exp Immunol 130: 449–458, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katoh M Trefoil factors and human gastric cancer (review). Int J Mol Med 12: 3–9, 2003. [PubMed] [Google Scholar]
- 26.Kim E, Gunther W, Yoshizato K, Meissner H, Zapf S, Nusing RM, Yamamoto H, Van Meir EG, Deppert W, Giese A. Tumor suppressor p53 inhibits transcriptional activation of invasion gene thromboxane synthase mediated by the proto-oncogenic factor ets-1. Oncogene 22: 7716–7727, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kodama M, Murakami K, Okimoto T, Sato R, Watanabe K, Fujioka T. Expression of mutant type-p53 products in H pylori-associated chronic gastritis. World J Gastroenterol 13: 1541–1546, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalani EN, Williams R, Jayaram Y, Gilbert C, Chaudhary KS, Siu LS, Koumarianou A, Playford R, Stamp GW. Trefoil factor-2, human spasmolytic polypeptide, promotes branching morphogenesis in MCF-7 cells. Lab Invest 79: 537–546, 1999. [PubMed] [Google Scholar]
- 29.Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol 9: 54–58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung WK, Yu J, Chan FK, To KF, Chan MW, Ebert MP, Ng EK, Chung SC, Malfertheiner P, Sung JJ. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J Pathol 197: 582–588, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Levine AJ p53, the cellular gatekeeper for growth and division. Cell 88: 323–331, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 13: 470–476, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SA, Christopherson KW, Bhat-Nakshatri P, Goulet RJ Jr, Broxmeyer HE, Kopelovich L, Nakshatri H. Negative regulation of chemokine receptor CXCR4 by tumor suppressor p53 in breast cancer cells: implications of p53 mutation or isoform expression on breast cancer cell invasion. Oncogene 26: 3329–3337, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Nakade K, Zheng H, Ganguli G, Buchwalter G, Gross C, Wasylyk B. The tumor suppressor p53 inhibits Net, an effector of Ras/extracellular signal-regulated kinase signaling. Mol Cell Biol 24: 1132–1142, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119: 847–860, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Ravi R, Mookerjee B, van Hensbergen Y, Bedi GC, Giordano A, El-Deiry WS, Fuchs EJ, Bedi A. p53-mediated repression of nuclear factor-kappaB RelA via the transcriptional integrator p300. Cancer Res 58: 4531–4536, 1998. [PubMed] [Google Scholar]
- 38.Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell 98: 141–152, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Salah Z, Haupt S, Maoz M, Baraz L, Rotter V, Peretz T, Haupt Y, Bar-Shavit R. p53 controls hPar1 function and expression. Oncogene 27: 6866–6874, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79: 639–646, 1999. [PubMed] [Google Scholar]
- 41.Seto E, Usheva A, Zambetti GP, Momand J, Horikoshi N, Weinmann R, Levine AJ, Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci USA 89: 12028–12032, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siu LS, Romanska H, Abel PD, Baus-Loncar M, Kayademir T, Stamp GW, Lalani el-N. TFF2 (trefoil family factor2) inhibits apoptosis in breast and colorectal cancer cell lines. Peptides 25: 855–863, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Wenger L, Rutter JL, Brinckerhoff CE, Cheung HS. Human metalloproteinase-1 (collagenase-1) is a tumor suppressor protein p53 target gene. Ann NY Acad Sci 878: 638–641, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Zeng XR, Wenger L, Firestein GS, Cheung HS. P53 down-regulates matrix metalloproteinase-1 by targeting the communications between AP-1 and the basal transcription complex. J Cell Biochem 92: 258–269, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Takaishi S, Wang TC. Providing AID to p53 mutagenesis. Nat Med 13: 404–406, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, Fleming JV, Takaishi S, Wang TC. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol 292: G1726–G1737, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci 47: 573–578, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Maehara Y, Oda S, Ichiyoshi Y, Kusumoto T, Sugimachi K. The p53 tumor suppressor gene in anticancer agent-induced apoptosis and chemosensitivity of human gastrointestinal cancer cell lines. Cancer Chemother Pharmacol 43: 43–49, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Vestergaard EM, Brynskov J, Ejskjaer K, Clausen JT, Thim L, Nexo E, Poulsen SS. Immunoassays of human trefoil factors 1 and 2: measured on serum from patients with inflammatory bowel disease. Scand J Clin Lab Invest 64: 146–156, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Weisz L, Zalcenstein A, Stambolsky P, Cohen Y, Goldfinger N, Oren M, Rotter V. Transactivation of the EGR1 gene contributes to mutant p53 gain of function. Cancer Res 64: 8318–8327, 2004. [DOI] [PubMed] [Google Scholar]