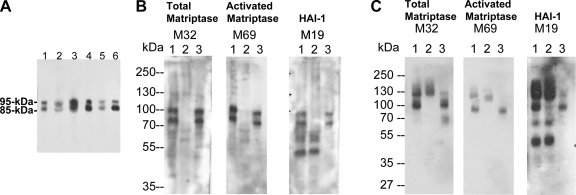
Fig. 1.
Secretion of matriptase-hepatocyte growth factor activator inhibitor (HAI-1) complexes, but not latent matriptase in seminal fluid and urine. A: matriptase in human seminal fluid. The presence of matriptase in human seminal fluid from six healthy donors was characterized by immunoblotting using the M32 mAb. Two major bands at around 85 kDa and 95 kDa were observed in all six specimens. B: seminal fluid contains activated matriptase in HAI-1 complexes, but no latent matriptase. The seminal fluid (lanes 1) was subjected to immunoprecipitation with immobilized matriptase mAb 21-9. The unbound fractions were collected (lanes 2), and the matriptase mAb-captured proteins were eluted using acidic buffer, pH 2.4, and then immediately neutralized (lanes 3). All of these samples were analyzed by immunoblotting for total matriptase using the mAb M32 (left, total matriptase, M32), activated matriptase using the mAb M69 (middle, activated matriptase, M69), and HAI-1 using the mAb M19 (right, HAI-1, M19). Both the 85- and the 95-kDa matriptase species were recognized by the activated matriptase mAb and the HAI-1 mAb. Noncomplexed HAI-1 fragment at 50 kDa was also detected by the HAI-1 mAb M19 (right, lane 1) and was not precipitated by matriptase mAb 21–9 beads. C: human urine contains matriptase-HAI-1 complex, but no latent matriptase. The matriptase species present in human urine were assessed by immunoprecipitation followed by immunoblotting through the same procedures as in B. All three mAbs identified the same two proteins bands: one at 95 kDa and a smeared band of >120 kDa (lanes 1). The 95-kDa protein band was depleted by precipitation with matriptase mAb 21-9 beads (lanes 2) and was recognized by the activated matriptase mAb M69 (middle, lane 3) and the HAI-1 mAb M19 (right, lane 3), suggesting that the 95-kDa species is matriptase-HAI-1 complex. The smeared protein bands that were identified in all three immunoblots were not precipitated using the matriptase mAb 21–9 beads (lanes 2), suggesting that the bands do not contain matriptase. The smear of bands is most likely the result of human immunoglobulins in the sample that are identified through cross-reaction with the anti-mouse IgG secondary antibody. A relatively sharp band present at the lower end of the smear (left, lane 1) is, however, immunodepleted by the matriptase mAb 21–9 beads (left, lane 2) and was eluted (left, lane 3). This 110-kDa matriptase band is the matriptase-antithrombin III complex described in our previous study (35). The 50-kDa, free HAI-1 was also detected in human urine by the HAI-1 mAb M19 (right, lane 1) but was not precipitated by the matriptase mAb 21–9 beads, as expected (right, lane 2).