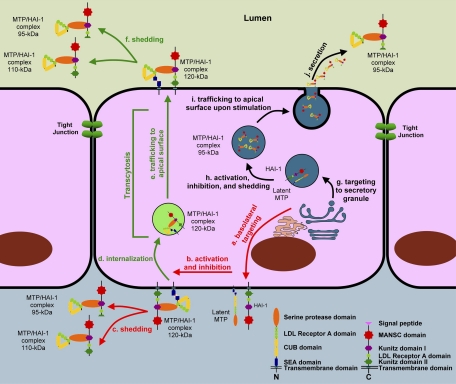
Fig. 10.
The models for matriptase activation, inhibition, and shedding. In most polarized epithelial cells, matriptase is synthesized as a latent zymogen and targeted to basolateral plasma membrane (a). Latent matriptase undergoes autoactivation to generate active matriptase, which is quickly inhibited by HAI-1 to form a 120-kDa complex (b). The 120-kDa matriptase-HAI-1 complex is either shed from the basal plasma membrane (c) or internalized (d) and then traffics to the apical plasma membrane (e) at which the 120-kDa complex is shed into the lumen as 95- and/or 110-kDa complexes by simultaneous proteolytic cleavages at both matriptase and HAI-1 (f). In specific types of epithelial cells, such as the gastric chief cells, matriptase may be synthesized and then targeted to secretory granules (g) within which matriptase undergoes autoactivation, HAI-1-mediated inhibition, and release as a 95-kDa complex with HAI-1, being liberated from the membrane through proteolytic cleavage (h). The 95-kDa matriptase-HAI-1 complex is stored in the secretory granules. Upon stimulation, the secretory granules traffic to apical surface (i), fuse with plasma membrane, and then release the 95-kDa matriptase-HAI-1 complex (j).