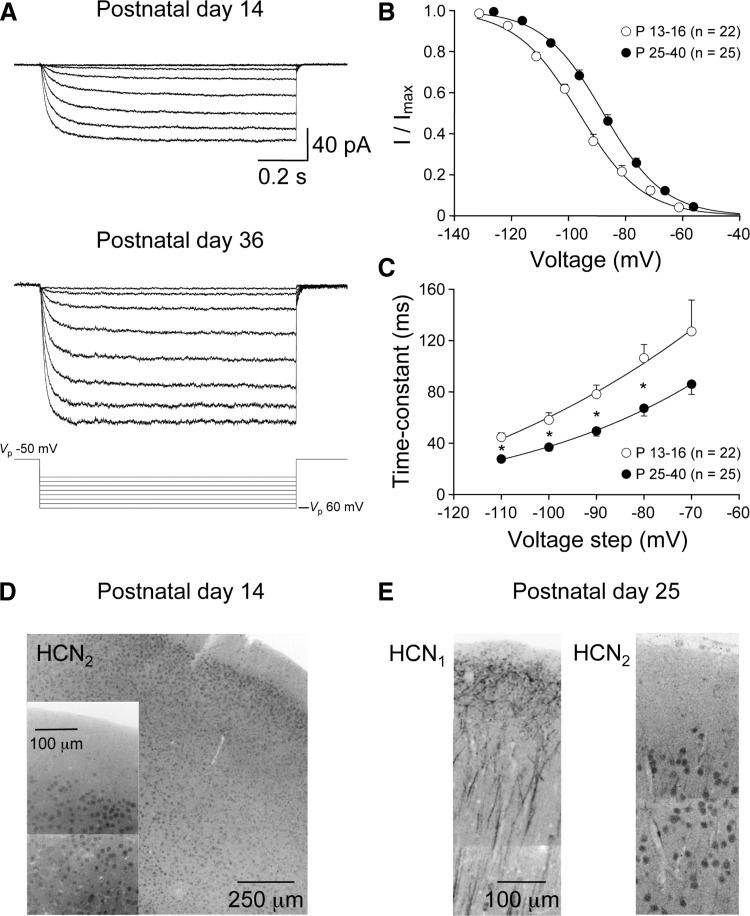
FIG. 8.
Developmental refinement of the activation properties of distal apical dendritic HCN channels. A: families of leak-subtracted cell-attached current responses evoked by an incremental series of voltage steps (bottom traces) recorded from an apical dendritic site of a P14 (70 μm from the layer 1–layer 2 border) and P36 layer 5 pyramidal neuron (30 μm from the layer 1–layer 2 border). Leak subtraction was performed off-line by the subtraction of scaled responses generated by interleaved 10-mV voltage steps. B: voltage-dependent activation curves of HCN channel activity calculated from tail currents in cell-attached patches made from young (P13-16, ○; means ± SE) and mature (P25–40, •) layer 5 pyramidal neurons. Voltage refers to calculated membrane potential (see results for details). Relationship have been fit with single Boltzmann functions (—). Note the relatively hyperpolarized voltage at half-maximal activation in the young group. C: activation kinetics of ensemble HCN channel activity are different between young and mature layer 5 pyramidal neurons across a wide voltage range. Note that in each group activation kinetics speed as the size of the pipette negative voltage step increases. Data are fit by single-exponential functions (—). The statistical significance between groups is indicated by stars (P values <0.01, 2-tailed t-test). D: HCN2 immunostaining (dark reaction) of the neocortex from a P14 animal; inset: the cell bodies of layer 2/3 neurons at a higher magnification. E: HCN1 and HCN2 immunostaining (dark reaction) in consecutive neocortical slices from a P25 animal. Note the prominent HCN1 immunostaining of the apical dendrites of layer 5 pyramidal neurons, and the somatic HCN2 immunostaining of layer 2/3 neurons. Montages were formed from a series of high-resolution confocal stacks.