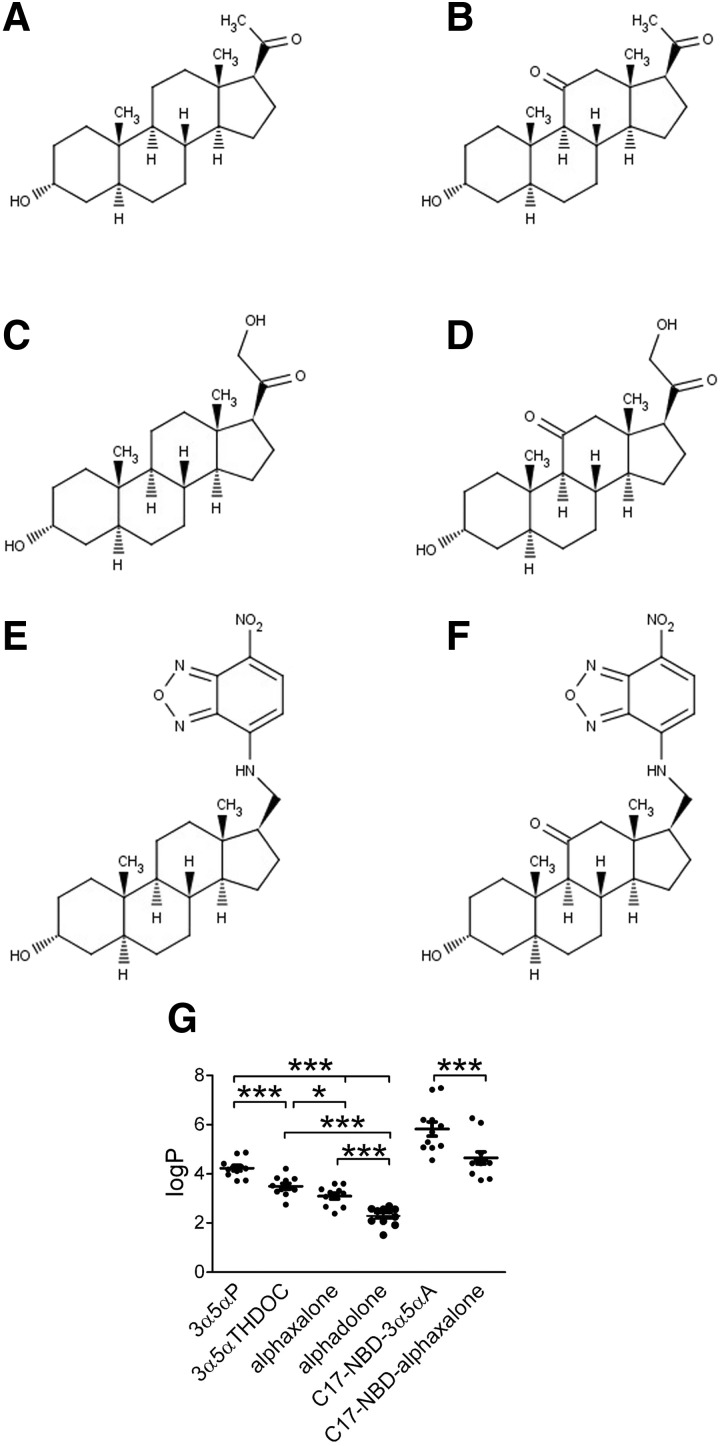
FIG. 1.
Structures and predicted lipophilicity of natural and synthetic steroid analogues that potentiate γ-aminobutyric acid type A (GABAA) currents. A: 3α5αP; B: alphaxalone; C: 3α5αTHDOC; D: alphadolone. 3α5αP and 3α5αTHDOC are natural neurosteroids. Alphaxalone and alphadolone are synthetic analogues. E and F: synthetic neuroactive steroid analogues with a fluorescent substituent (NBD) at carbon 17, C17-NBD-3α5αA (E) and C17-NBD-alphaxalone (F). G: estimated partition coefficients (log P) for each steroid. Values were calculated using 11 different algorithms and are shown as means ± SE, *P < 0.05, ***P < 0.0001 (paired t-test). Alphadolone, (3α,5α)-3,21-dihydroxypregnane-11,20-dione; alphaxalone, (3α,5α)-3-hydroxypregnane-11,20-dione; 3α5αP, (3α,5α)-3-hydroxypregnan-20-one; 3α5αTHDOC, (3α,5α)-3,21-dihydroxypregnan-20-one; NBD, 7-nitrobenz-2-oxa-1,3-diazole.